Glen Report 5-12: Psoralen C2 / Direct Fluorescein Labelling of Oligonucleotides
DIRECT FLUORESCEIN LABELLING OF OLIGONUCLEOTIDES
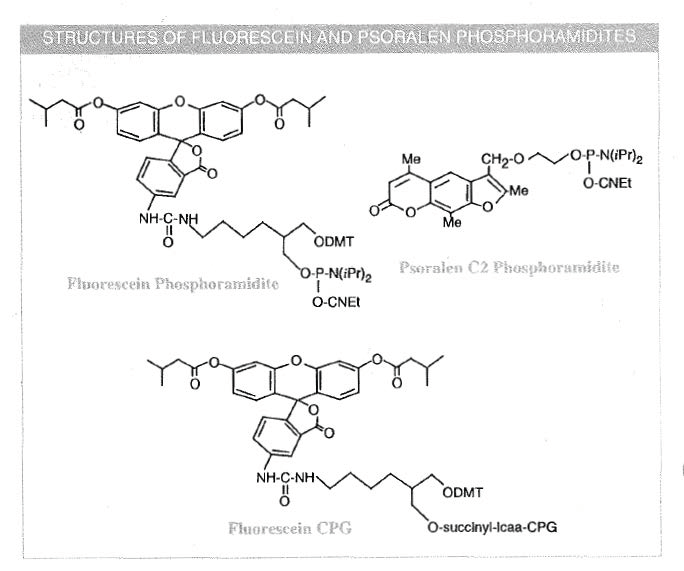
After biotin labelling, the label most often requested has been fluorescein for-use in both diagnostic development and sequencing applications. Synthesis of fluorescein labelled sequencing primers is likely to be the primary use for a fluorescein phosphoramidite: A phosphoramidite based on the methyl ester of fluorescein has been described 1 . However, we preferred to design a phosphoramidite which would produce, on deprotection and isolation of the derived oligonucleotide, the same fluorescein-type structure as had been previously prepared using fluorescein isothiocyanate (FITC). Our fluorescein phosphoramidite also contains a DMT group to allow quantification of coupling.
Fluorescein phosphoramidite is used in a manner identical to normal nucleoside phosphoramidites. We recommend that the synthesis be carried out DMT-off to allow the determination of the level of fluorescein labelling. Oligonucleotides are deprotected with ammonium hydroxide as normal; the protecting pivaloyl groups are removed and the linkage to fluorescein is stable under these conditions. Fluorescein labelled oligonucleotides may be purified by gel electrophoresis or HPLC. However, the product, when used for labelling at the 5'-terminus, is designed to be compatible with reverse phase purification techniques, including disposable cartridges. We recommend following the normal DMT-on cartridge purification procedure but omitting the 2% TFA wash (which normally removes the DMT group). The product can be eluted with aqueous acetonitrile to remove all fluorescence from the cartridge (1 - 2 mL is usually sufficient).
Reference
1) F. Schubert, K. Ahlert, D. Cech, and A. Rosenthal, Nucleic Acids Res., 1990, 18, 3427.
Product Information
Fluorescein Phosphornmidite (10-1963)
Fluorescein CPG (20-2963)
Psoralen C2 Phosphoramidite (10-1982)
- Glen Report 5-11: 2'-0Me-RNA -The Logical RNA Alternative?
- Glen Report 5-12: Psoralen C2 / Direct Fluorescein Labelling of Oligonucleotides
- Glen Report 5-13: BioTEG - The Ultimate Biotin Phosphoramidite
- Glen Report 5-14: Blocking the Termini of Oligonucleotides
- Glen Report 5-15: Thiol Modification of Oligonucleotides
- Glen Report 5-16: Spacer Phosphoramidite
- Glen Report 5-17: Unusual Monomers

