Glen Report 37-16: Literature Highlight - Highly-modified mRNA Capture Sequences
At Glen Research, we have a large catalog of products for oligonucleotide synthesis. Depending on their needs, customers will determine which products to use as well as where to place them in a sequence. This can result in some very complex oligonucleotides, as was the case in an ACS Chemical Biology article from a few years ago.1
The authors were working to optimize an in vivo, single cell transcriptome analysis assay that the same group described in earlier publications.2 3 The final mRNA capture sequence (Figure 1) had a total of 12 different products incorporated, six of which were non-nucleosidic modifications. There was a thiol modifier that was conjugated to a cell penetrating peptide (CPP) to facilitate cellular uptake. There was of course a poly-U sequence that is used to capture the poly-A tails of mRNA. That region was blocked with a photo-cleavable cage of two segments of A’s held together with a spacer and two photocleavable linkages. There was also a Cyanine 5 / Cyanine 3 FRET pair that can be used to monitor both cellular uptake as well as photo-decaging. Finally, there was a biotin to facilitate capture of the desired mRNA sequences onto streptavidin. A full list of the products used can be found on the next page (Table 1).
Figure 1. mRNA capture sequence
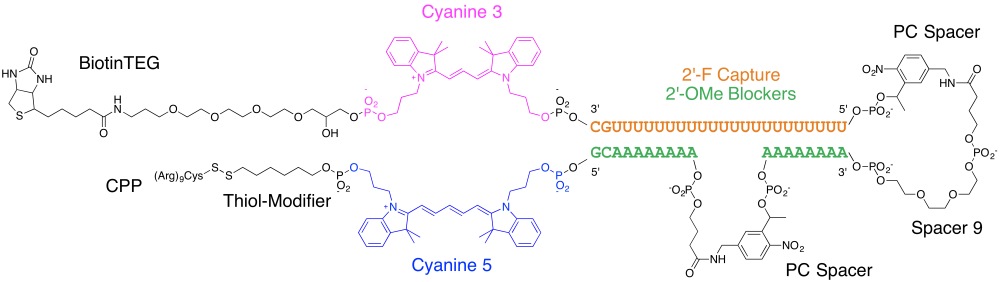
As one can imagine, synthesizing such an oligonucleotide requires a bit of planning. First and foremost, the synthesizer may not have 11 phosphoramidite ports, as was the case for the authors who were using an ABI394. That meant that the synthesis had to be performed over at least two runs. One must also consider how the deprotection needs to be done. Of the modifications present, the Cyanine 5 is the most deprotection-sensitive. We usually recommend an UltraMild synthesis, but since UltraMild versions of the 2'-F phosphoramidites are not available, deprotection with ammonium hydroxide at room temperature was used instead. Purification was performed with RP-HPLC, which allows for simultaneous monitoring of UV as well as the absorbances of the Cyanine 5 and Cyanine 3.
This is just one example of what our customers can do with our products, and we look forward to seeing many more examples in the future.
Table 1. Products used for mRNA capture sequence
| Product | Catalog No. |
| 3'-BiotinTEG CPG | 20-2955 |
| Cyanine 3 Phosphoramidite | 10-5913 |
| 2'-F-Ac-C-CE Phosphoramidite | 10-3415 |
| 2'-F-G-CE Phosphoramidite | 10-3420 |
| 2'-F-U-CE Phosphoramidite | 10-3430 |
| PC Spacer Phosphoramidite | 10-4913 |
| Spacer Phosphoramidite 9 | 10-1909 |
| 2'-OMe-A-CE Phosphoramidite | 10-3100 |
| 2'-OMe-Ac-C-CE Phosphoramidite | 10-3115 |
| 2'-OMe-G-CE Phosphoramidite | 10-3121 |
| Cyanine 5 Phosphoramidite | 10-5915 |
| Thiol-Modifier C6 S-S | 10-1936 |
References
- S.B. Yeldell, L. Yang, J. Lee, J.H. Eberwine, and I.J. Dmochowski, ACS Chem Biol, 2020, 15, 2714-2721.
- D. Lovatt, et al., Nat Methods, 2014, 11, 190-196.
- S.B. Yeldell, B.K. Ruble, and I.J. Dmochowski, Org Biomol Chem, 2017, 15, 10001-10009.
- Glen Report 37-11: New Product- N3-Methyl-C-CE Phosphoramidite
- Glen Report 37-12: Product Review - Post-transcriptional RNA Modifications
- Glen Report 37-13: New Product Ac-5-Me-C-LA-CE Phosphoramidite
- Glen Report 37-14: Application Note - Enzymatic Recognition of Nucleic Acid Modifications
- Glen Report 37-15: New Product - Amino-Modifier C6 dR
- Glen Report 37-16: Literature Highlight - Highly-modified mRNA Capture Sequences
- Glen Report 37-17 Application Note - Aptamers in Diagnostics
- Glen Report 37-18 Technical Snippets

