Glen Report 28.26: Transient Cyanoethylation - An Unexpected Route to Chemical Bleaching of a Fluorophore
As a chemist, reading that the use of a fast-cleaving support could lead to degradation of the AquaPhluor 593 should leave you scratching your head - how could a distal linker that attaches the dye to the support affect the stability of the dye? And why would treatment with a hindered base, such as diethylamine prior to deprotection in ammonia, somehow protect the dye when neither the dye nor the linker are directly affected by the DEA? The answer to this is surprising - transient cyanoethylation.
Cyanoethylation is a commonly seen reaction during the deprotection of chemically synthesized DNA. The phosphodiester backbone is essentially ‘protected’ as a cyanoethyl phosphotriester. Upon treatment with base, the cyanoethyl group is eliminated to form acrylonitrile, yielding the normal phosphodiester backbone. However, acrylonitrile is a good electrophile and if nucleophiles such as thiols or amines are present, they can react with the acrylonitrile via a Michael addition to yield a cyanoethylated product. This cyanoethylation can be especially problematic on large-scale syntheses because, at a high enough concentration, the acrylonitrile will react at the N3-position of thymidine. This is one of the reasons that deprotection using AMA (Ammonium hydroxide/Methylamine) is popular - not only does it deprotect the DNA strand quickly, it also suppresses the cyanoethylation of thymidine due to its being a strong nucleophile that readily reacts with acrylonitrile. When AMA is not compatible with either the bases or modifiers in the oligo, then a pre-treatment with 10% diethylamine in ACN is a common practice to prevent cyanoethylation. In this case, however, the cyanoethylation appears to be occurring on the oxygen of the xanthene dye - but only transiently as shown in the mechanism in Figure 1.
Figure 1: Mechanism of Cyanoethylation Reaction on AquaPhluor® 593
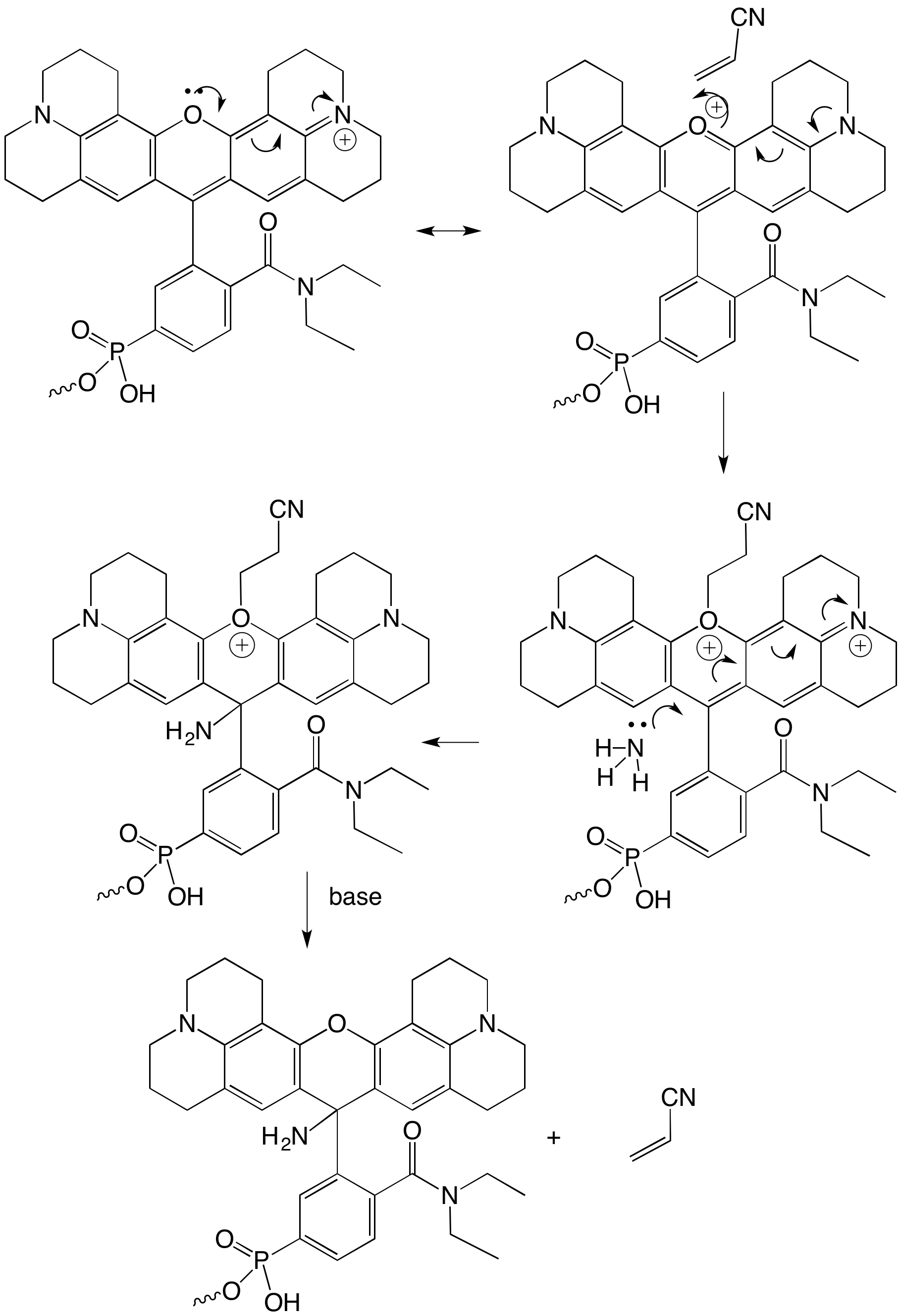
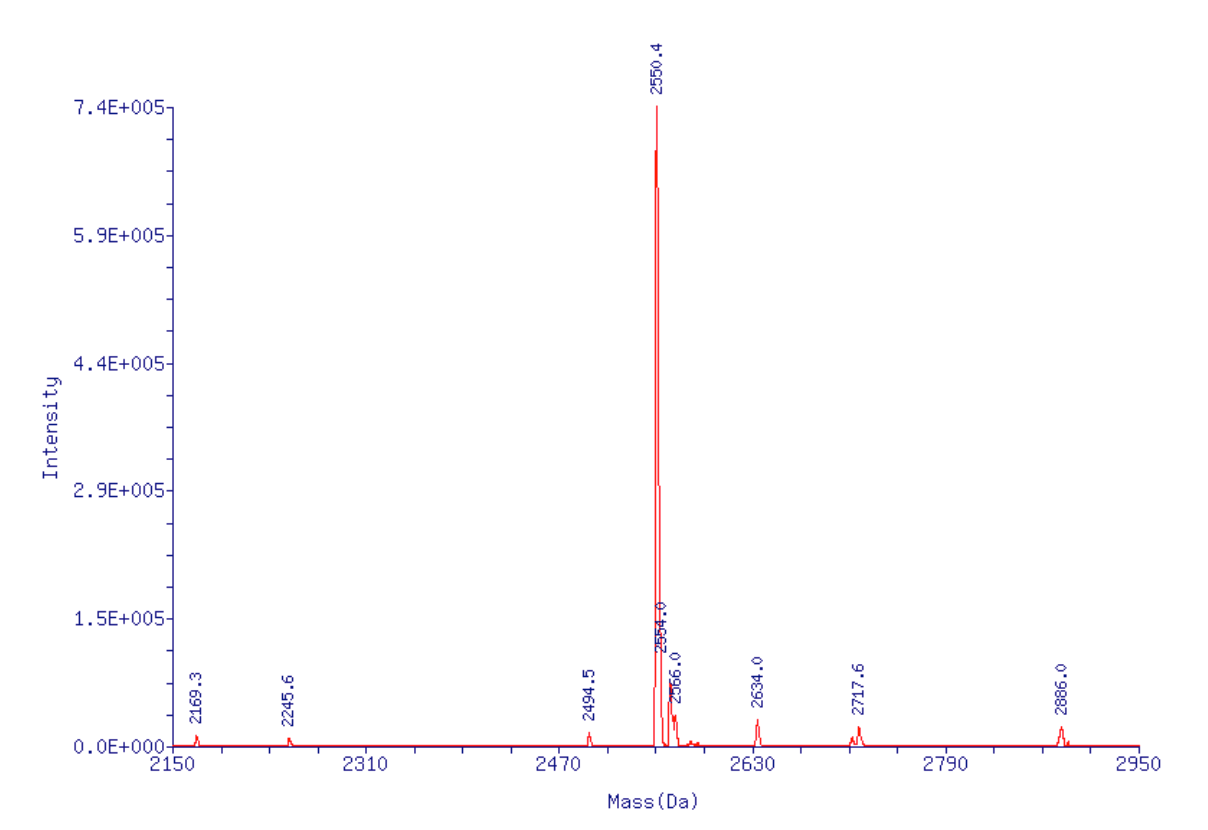
More often than not, the transient cyanoethylation is quickly eliminated and the dye is left intact. However, the cyanoethylation does set up the ring for nucleophilic attack. If this occurs with NH3 while the oxygen is cyanoethylated, then the you get an irreversible addition of NH2 to the molecule, interrupting aromaticity and killing the dye. The cyanoethyl group is later eliminated, so you end up with a +16 Da species due to the addition of NH2. Sure enough, as shown in Figure 2, the ESI MS performed on AP593-T6 with a target mass of 2551.0 (observed mass 2550.4), shows the presence of the amine-adduct with a target mass of 2567.0 (observed mass 2566.1), confirming the presence of the predicted impurity. (Note the other peaks are a series of HFIPA adducts and deconvolution artifacts). To confirm this, the oligo AP593-T6, previously deprotected and purified so there was none of the degradation product, was added to ammonium hydroxide and then T20-CPG was added - the equivalent of 1 umole of a T20. After 2 hr at 65 °C, the oligo was analyzed and as predicted, the non-fluorescent impurity was observed.
Figure 2: ESI MS of 5'-AquaPhluor® 593 T6 prepared using dT-Q-CPG
When it was reported to our collaborators at Epoch Biosciences that chemical bleaching of the AP593 fluorophore was observed as a side-reaction when deprotecting in ammonium hydroxide, they were surprised. It wasn’t something they had observed before. We repeated our experiments here as did they making the same sequence, deprotecting under the same conditions and analyzing using the same method by RP HPLC. Here, approximately 11% of a non-fluorescent compound was again observed and there (again) it was not. However, there was a difference - the support used. We used a fast-cleaving dT-Q-CPG and they used a standard dT support with a succinate linker.
When testing new products here, we often choose to use the Q-support that Richard Pon developed some years ago. On a dT-Q support, the oligo is cleaved off at room temperature in ammonium hydroxide within 2-3 minutes whereas a standard succinate support takes a couple of hours. By using the Q-support, one can cleave off the oligo and analyze it without any degradation to a minor base or modifier, giving you a time zero when determining the kinetics of deprotection or degradation.
But why would a fast-cleaving support lead to more cyanoethylation of the dye and subsequent degradation? - because the acrylonitrile released upon addition of the ammonium hydroxide quickly diffuses away from the still-bound oligo and into the solution above it. This essentially lowers the local concentration of acrylonitrile near the CPG-bound oligo, lowering its rate of reaction. By the time the oligo is cleaved, the acrylonitrile has been deactivated by a Michael addition by NH3 and therefore the side-reaction is limited. However, when using a fast-cleaving support, the oligo is quickly cleaved and goes into solution along with the acrylonitrile, allowing it to react and degrade the dye - all due to an unexpected transient cyanoethylation - which, happily, can be prevented simply by treatment with hindered base.
Product Information
5'-AquaPhluor® 593 CE Phosphoramidite (10-5923)
5'-AquaPhluor® 593 CPG (20-5923)
- Glen Report 28.21: Versatile Applications of the Copper(I)-Catalyzed Click Chemistry
- Glen Report 28.22: New Product - TIPS-5-Ethynyl-dU-CE Phosphoramidite
- Glen Report 28.23: Technical Brief – Fluorescein Labelling – Considering the Options
- Glen Report 28.24: New Products – AquaPhluor® 593 5’-Phosphoramidite and CPG
- Glen Report 28.25: New Product: Glen-Pak™ DNA 3mg 384-Well Plate
- Glen Report 28.26: Transient Cyanoethylation - An Unexpected Route to Chemical Bleaching of a Fluorophore

