Glen Report 28.23: Technical Brief – Fluorescein Labelling – Considering the Options
Figure 1: Structures of Fluorescein Phosphoramidites and Supports |
|
 |
 |
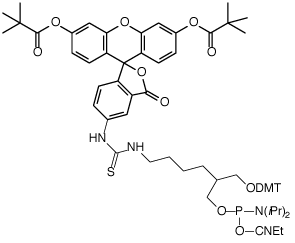
(1) Fluorescein Phosphoramidite (10-1963) |
(2) 3’-Fluorescein CPG (20-2963) |
 |
 |
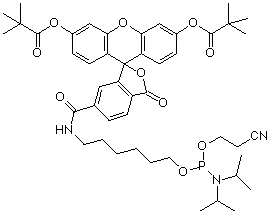
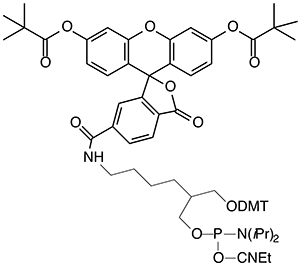
(3) 5’-Fluorescein Phosphoramidite (10-5901) |
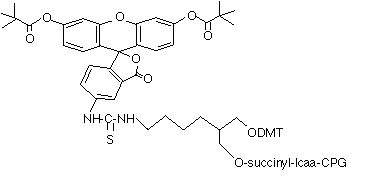
(4) 3’-(6-FAM) CPG (20-2961) |
 |
 |
(5) 6-Fluorescein Phosphoramidite (10-1964) |
(6) 3’-(6-Fluorescein) CPG (20-2964) |
 |
 ; ; |
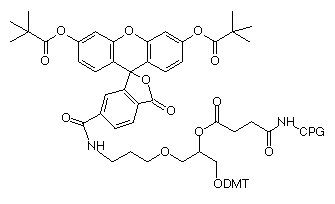
(7) 6-Fluorescein Serinol Phosphoramidite (10-1994) |
(8) 3’-6-Fluorescein Serinol CPG (20-2994) |
Fluorescein may not be the ideal label for oligonucleotides due to the pH dependence of its fluorescence (fluorescent only at pH >7) and its tendency to photobleach. Regardless, fluorescein remains one of the most popular labels for oligonucleotides. Over many years, Glen Research has accumulated a wide variety of products for fluorescein labelling and, in this article, we will describe the pros and cons of our various options for 3’ and 5’ labelling.
In the early days, the most popular reagent for fluorescein labelling in general was fluorescein isothiocyanate (FITC), so it was natural for us to offer products for ‘FITC labelling’ optimized for oligonucleotide synthesis. Our first products were the FITC derived phosphoramidite (1) and CPG (2), based on the 1,3-diol backbone developed by Nelson.1 Both of these products remain popular and allow very efficient fluorescein labelling. However, as mass spectrometric analysis became more popular, it became clear that there was an inherent flaw in these products. The thiourea linkage derived from FITC is not stable2 to deprotection conditions and is susceptible to side reactions, including hydrolysis to a urea linkage and ammonolysis to a guanidine linkage, as shown in Figure 2. While these side reactions have no effect on the fluorescein content, they do complicate the MS analysis and potentially RP HPLC analysis of singly and especially doubly labelled oligonucleotides.
Soon thereafter, 5’-fluorescein phosphoramidite (6-FAM) (3) was introduced and this product was prepared using 6-carboxyfluorescein as a single isomer. 6-FAM rapidly became the most popular fluorescein labelling product since it simply generates a pure oligonucleotide labelled at the 5’ terminus. So, it was natural for us to apply the 6-FAM approach to our branched product line and we introduced 6-fluorescein phosphoramidite (5) and the equivalent 3’-(6-fluorescein) CPG (6). These two products offer the benefits of 6-FAM (simplicity, isomeric purity and minimal side reactions) along with the ability to purify derived oligos by Glen-Pak™ DMT-ON purification.
Many years ago, we also introduced 3’-(6-FAM) CPG (4), which is based on the well used 1,2-diol backbone. We still offer this product and its popularity has never waned. However, as we have demonstrated in past newsletters, this type of backbone is susceptible to an elimination reaction during deprotection that leads to loss of label.
More recently, we introduced 6-fluorescein serinol phosphoramidite (7) and the equivalent 3’-6-fluorescein serinol CPG (8) based on our increasingly popular serinol-based 1,3-diol linkage.3 These products offer equivalent performance levels to phosphoramidite (4) and CPG (5).
Experimental Design
In order to take a close look at the performance of these eight fluorescein products, we have carried out a simple set of experiments designed to illustrate the strength and weakness of each product.
In the case of the phosphoramidites, each was added to the 5' terminus of a simple T6 oligo. Where apropriate, the final DMT group was removed and the crude oligo was analyzed by reverse phase HPLC.
In the case of each support, a T6 oligonucleotide was synthesized followed by deprotection and DMT-ON purification using Glen-Pak cartridges. The DMT-ON purification was designed to remove any early failures so that we could focus entirely on the 3' linkage to fluorescein.
In all cases, the oligonucleotides were deprotected using ammonium hydroxide at 55 °C for 17 hours to closely mimic the deprotection of regular oligonucleotides.
Results
5' Terminus
As expected, the 5'-fluorescein phosphoramidite (3) performed impeccably, yielding a product oligonucleotide, which was essentially 100% pure. The results for 6-Fluorescein, as shown in Figure 3a, and 6-Fluorescein Serinol Phosphoramidites were the same at close to 100% purity. However, the results for Fluorescein Phosphoramidite looked even worse than we had expected. The chromatogram showed several impurity peaks but the UV/Visible spectrum of all components confirmed the presence of fluorescein, as shown in Figure 3b.
3'-Terminus
Oligonucleotide synthesis with the label at the 3' terminus is much more difficult than at the 5' terminus. The label itself is challenged by all of the reagents of oligonucleotide synthesis in multiple cycles. Similarly, the linker is susceptible to side reactions.
Figure 2: HYDROLYSIS OF C=S Hydrik

In our experiments, three of the supports, 3'-(6-Fluorescein), 3'-(6-FAM) and 3'-6-Fluorescein Serinol performed very well with only minor side reactions being observed at the linker. One surprise for us was that 3'-(6-FAM), attached by a 1,2-diol based linker and perfectly set up for an elimination reaction, did not lead to any appreciable loss of label in this experiment, as shown in Figure 3c.
On the other hand, 3'-Fluorescein exhibited even more side reactions at the thiourea linker than expected, as shown in Figure 3d, but, again, all peaks contained fluorescein. In addition to the expected hydrolysis and ammonolysis side products illustrated in Figure 2, mass spectroscopic analysis showed there were also components in the mixture with additional sulfur in the oligos. This may indicate that the hydrolytic mechanisms leading to loss of sulfur can also cause sulfurization of adjacent phosphodiester linkages to yield a family of phosphorothioate modifications.
Figure 3: RP HPLC of Fluorescein Containing Oligos

Conclusion
All of our fluorescein products performed well in these tests. In particlular, 6-Fluorescein phosphoramidite (10-1964) and CPG (20-2964) and 6-Fluorescein Serinol (10-1994) and CPG (20-2994) proved to be very strong performers as phosphoramidites and supports, with minimal side reactions related to their 1,3-diol based linkers.
One surprise was the very high quality of product derived from 3'-(6-FAM) CPG (20-2961). In this experiment, we did not observe any significant elimination of the 3' linkage with attendant loss of fluorescein.
However, it is clear that the thiourea linker derived from FITC in Fluorescein Phosphoramidite (10-1963) and CPG (20-2963) should not be used for any more complex experiments than simple 5' or 3' labelling.
References
1. P.S. Nelson, M. Kent, and S. Muthini, Nucleic Acids Research, 1992, 20, 6253-6259.
2. I. Dubey, G. Pratviel, and B. Meunier, Bioconjug Chem, 1998, 9, 627-32.
3. US Patent No.: 8,394,948 (https://www.glenresearch.com/reports/gr25-14)
Product Information
- Glen Report 28.21: Versatile Applications of the Copper(I)-Catalyzed Click Chemistry
- Glen Report 28.22: New Product - TIPS-5-Ethynyl-dU-CE Phosphoramidite
- Glen Report 28.23: Technical Brief – Fluorescein Labelling – Considering the Options
- Glen Report 28.24: New Products – AquaPhluor® 593 5’-Phosphoramidite and CPG
- Glen Report 28.25: New Product: Glen-Pak™ DNA 3mg 384-Well Plate
- Glen Report 28.26: Transient Cyanoethylation - An Unexpected Route to Chemical Bleaching of a Fluorophore

