Glen Report 27.16: Technical Brief - Capping and Trityl-Protected Amino-Modifiers
Two of our most popular products are the trityl-protected amino-modifiers, 10-1906 (1) and 10-1907 (2) in Figure 1. These products are particularly useful for two reasons. First, the trityl protecting group on the amine allows for simple reverse-phase purification by HPLC or cartridge to yield a clean, full-length product. Second, the trityl protecting group can be removed from the amine on the DNA synthesizer, which allows the amine to be labelled while the oligo is still bound to the CPG. This eliminates the need for any tedious desalting steps to remove unconjugated NHS ester label. However, we recently determined these products can undergo an unexpected side-reaction that leads to capping of the amine.
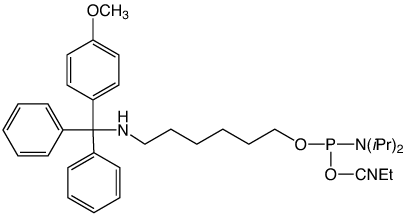
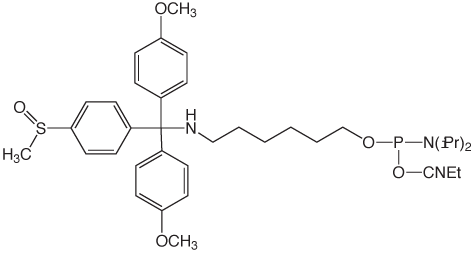
A customer shared with us an observation that his oligo had an unusual +190 Da peak as seen by mass spectrometry (MS). This mass corresponds to the addition of t-Bu-phenoxyacetyl to the amine and an UltraMild Cap A mix, which contains t-Bu-phenoxyacetic anhydride, was indeed used on the synthesizer. The amino-modifier in question was the 5'-DMS(O)MT-Amino-Modifier C6 (2). The trityl on this amino-modifier, 4,4'-dimethoxy-4"-methylsulfonyl-trityl, is the most acid-labile used in any of the amino-modifiers due to the exceptional stability of the DMS(O)MT trityl cation. This lability, perhaps, facilitated the phenoxyacetylation of the amine during the capping step. While we had not received reports of acetylation of the amino-modifier protected with the DMS(O)MT, there was the possibility of some unexpected chemistry occurring when phenoxyacetic anhydride was used as the capping reagent during synthesis.
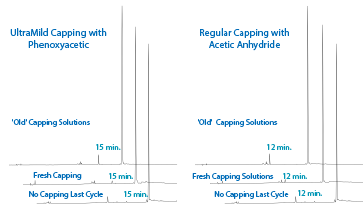
To investigate this possibility, the sequence 5'-X-TTT TTT-3', where X is the DMS(O)MT Amino-Modifier C6, was synthesized with the capping steps in the synthesis cycle disabled. The CPG was split, capping one portion off the synthesizer with standard Cap A/B, which uses acetic anhydride, and the other with UltraMild Cap A/B which uses phenoxyacetic anhydride. The samples were then cleaved from the support in ammonium hydroxide and analyzed by RP HPLC. If capping had occurred, the Trityl-On peaks would be reduced and capped-amine T6 peaks would arise. As seen in Figure 2, the chromatogram of the oligo capped with the phenoxyacetic anhydride Cap A shows a sharp peak that elutes around the 15 minute mark with failures and the trityl-on amino-T6 eluting at 12 and 19 minutes, respectively. However, surprisingly, a new peak arose with the acetic anhydride-capped oligo at 12.5 minutes that corresponds to the acetyl-capped amine-T6.
Adapted from the mechanism determined for the interaction of pseudouridine synthase I with 5-fluorouracil-tRNA1
So, the experiment was repeated, this time using fresh, unopened bottles of both the standard Cap A and the UltraMild Cap A and Cap B which contains 16% methyimidazole. As seen in Figure 2, the respective acetyl and phenoxyacetyl capped species are still present, however at a much lower amount.
Our hypothesis to explain these data is that the old capping reagents become more and more acidic over time as the acetic- and phenoxyacetic anhydride hydrolyzes. This in turn results in the loss of the trityl group during capping and gives rise to N-acetyl (+42 Da) and N-phenoxyacetyl (+190 Da) species. Our results indicate that this is a more significant issue with 5'-DMS(O)MT-Amino-Modifier C6 (2), due to its greater lability to acid compared to 5'-Amino-Modifier C6 (1).
However, this side-reaction can be avoided by simply maintaining fresh capping reagents on the synthesizer. It is tempting to pull specialty capping reagents, e.g., UltraMild Cap A, off the synthesizer to be stored until the next time a synthesis that requires UltraMild Cap A comes up. However, these data show that this procedure is not without risk of side reactions. The results also confirm that an even simpler approach is to omit the capping step in the final cycle when using 5'-modifiers that terminate the oligo synthesis.
Reference
Ordering Information
5'-Amino-Modifier C6 (10-1906)
5'-DMS(O)MT-Amino-Modifier C6 (10-1907)
- Glen Report 27.11: Reversible Photo-Switching of DNA Function with Azobenzene-Tethered DNA
- Glen Report 27.12: New Products - Dithiol Serinol Phosphoramidite and 3’- Dithiol Serinol CPG
- Glen Report 27.13: Technical Brief - APA: An Alternative to AMA Deprotection
- Glen Report 27.14: Photocleavable Biotin Linker for Use in SOMAscan™
- Glen Report 27.15: PC Modifiers
- Glen Report 27.16: Technical Brief - Capping and Trityl-Protected Amino-Modifiers
- Glen Report 27.17: Technical Brief - DBCO-dT - An Unusual Case of Iodine Sensitivity

