Glen Report 15.15: A Novel Route to Activated Carboxylate Modified Oligonucleotides
Most conjugation schemes start with the amino or thiol group on the oligonucleotide and require that the molecule to be conjugated is first converted to either an active ester for amine reactions, such as a succinimide, or a thiol reactive maleimide. In either case, it is very costly to conduct a screen of a wide number of different conjugates. Also, it is not always possible to find a convenient starting material in order to prepare an activated ester. In some cases, the molecule is not even amenable to activation because of other properties that interfere with the chemistry. Another option is to react the oligonucleotide with either a heterobifunctional or homo-bifunctional linker. This broadens the applicability by allowing conjugation with other types of molecules but adds steps to the process and is often difficult to reproduce.
An alternative approach is to place the active ester on the oligonucleotide and conjugate with a molecule containing a primary amine. A wider range of unusual amines is readily available and generally more affordable. If the amine component is stable to the mild base used for deprotection, then it can be used with carboxy-modified oligo-nucleotides.
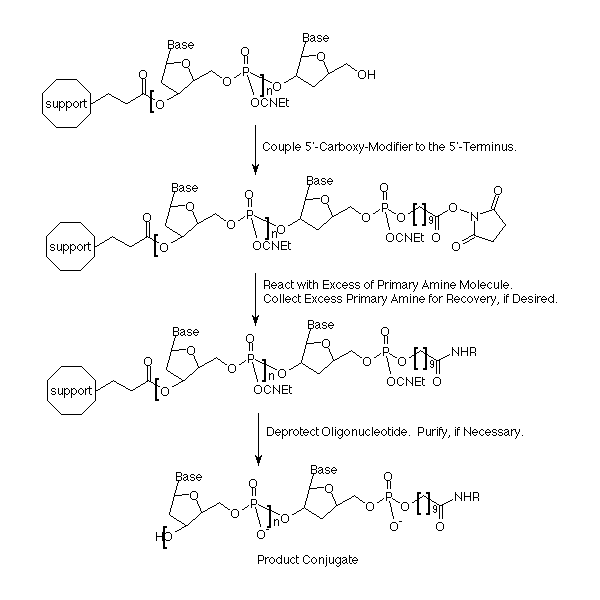
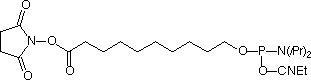
Glen Research is pleased to add 5'-Carboxy-Modifier-CE Phosphoramidite (Figure 2) to our range of products for the preparation of modified oligonucleotides. This unique linker is designed to be added at the terminus of an oligonucleotide synthesis. It contains an activated carboxylic acid N-hydroxysuccinimide (NHS) ester suitable for immediate conjugation with molecules containing a primary amine, resulting in a stable amide linkage. After the support has been removed from the DNA synthesis column, the molecule with the amino group is dissolved in an organic solvent and reacted with the NHS ester. Excess amino compound can be flushed from the column and recovered, if necessary. The oligonucleotide is then deprotected by a method appropriate for the reactivity of the modified oligonucleotide.
5'-Carboxy-Modifier C10
This procedure offers a novel way of preparing conjugates with much more flexibility and was originally designed to allow higher throughput screening of a large number of conjugates. The process using 5'-Carboxy-Modifier is shown in Figure 1. The modifier is coupled to the terminus, usually at the 5' end of an oligonucleotide. The linker is now ready to be coupled to any primary amine. This greatly increases the number of conjugates that may be readily prepared. Now, researchers can quickly screen a large number of interesting conjugates that were chemically difficult, if not impossible, before, and do it in a controlled, single step fashion that enhances the probability of success.
The fact that the conjugation chemistry is accomplished while the oligonucleotide is still support-bound adds several advantages. Often, successful conjugations are limited to those that are amenable to the requirement that some water (20-50%) is needed to dissolve the oligonucleotide in the conjugation mixture. Solid phase conjugations allow the use of completely organic systems, even dichloromethane, for conjugation. This allows the ready conjugation of very lipophilic compounds to oligonucleotides, which can then act as purification handles for reverse phase purification of the conjugates.
Another advantage of this system is that the reagent used in excess, the amine, is not affected by the reaction and therefore recoverable. In fact, the reagent can be used again without further purification for the next conjugation reaction. Besides the obvious cost savings, this will also improve overall efficiency in that much larger excesses are now feasible even in large-scale conjugations. By driving the reaction further to completion, downstream operations such as purifications are reduced or even eliminated.
5'-Carboxy-Modifier C10 is offered for sale under license from TriLink BioTechnologies, Inc. It is intended for research and development purposes only, and may not be used for commercial, clinical, diagnostic or any other use. It is covered under US Patent No. 6,320,041.
Sample Applications
1. Conjugation of an Amino Compound
- Couple 5'-Carboxy-Modifier C10 to the oligonucleotide using any conventional cycle. Do NOT allow the synthesizer to proceed to normal cleavage with ammonium hydroxide.
Remember that conjugation must occur before deprotection using most standard conditions, especially those using ammonium hydroxide or methylamine. Ammonium hydroxide will lead to the amide and methylamine will form the methylamide. - Wash the support with acetonitrile and air dry it. Add the support to a vial containing 10 equivalents of the amine dissolved in 2 mL of dichloromethane or other appropriate solvent with 10% triethylamine. Leave the reaction at room temperature for 4 hours with continuous agitation. Decant the amine solution from the support and wash with further dichloromethane. Air dry the support.
- Proceed to regular deprotection, keeping in mind the stability of the modified oligonucleotide to the deprotection conditions.
2. Preparation of Oligonucleotides with 5' Carboxylic Acid Linkers
- Couple the 5'-Carboxy-Modifier-CE Phosphoramidite to the oligonucleotide using any conventional cycle. Do NOT allow the synthesizer to proceed to normal cleavage with ammonium hydroxide.
- Deprotect the oligonucleotide overnight at room temperature using 0.4 M methanolic sodium hydroxide (Methanol/Water 4:1)
Note: NaOH is not compatible with dmf protecting groups. - Isolate the oligonucleotide by first passing the reaction through the desalting process of your choice, and then purify as usual. The product may also be used crude after desalting.
Product Information
- Glen Report 15.11: Redmond Red™ and Yakima Yellow™ dyes, and Eclipse™ Non-Fluorescent Quencher
- Glen Report 15.12: Comparison of Deprotection Methods for 3'-PT-Amino-Modifier CPG
- Glen Report 15.13: Pyrrolo-C - A Novel Fluorescent Nucleoside
- Glen Report 15.14: More and Still More Novel Minor Bases
- Glen Report 15.15: A Novel Route to Activated Carboxylate Modified Oligonucleotides