Glen Report 15.12: Comparison of Deprotection Methods for 3'-PT-Amino-Modifier CPG
A few months ago, we introduced the new 3-Amino-Modifier supports, shown in Figure 1, (1) and (2). In this alternative approach1, the nitrogen destined to become the 3-amino group is included in a phthalimide (PT) group which is attached to the support through an amide group on the aromatic ring. This simple linkage is very stable to all conditions of oligonucleotide synthesis and contains no chiral center. In the last Glen Report2, we compared yields and purity of crude oligonucleotides produced with 3-Amino-Modifier C7 CPG (3) and 3-PT-Amino-Modifier C6 CPG (2). The results indicated that the yield of product from the 3'-PT-Amino-Modifier C6 CPG is about 20% lower if deprotected with ammonium hydroxide. However, the purity of amino-modified product is significantly higher due to the absence of the acetyl capped product.
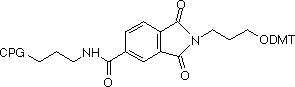 |
| (1) 3-PT-Amino-Modifier C3 CPG |
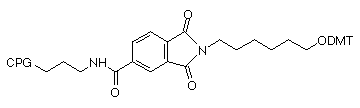 |
| (2) 3-PT-Amino-Modifier C6 CPG |
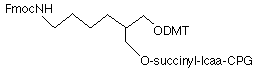 |
| (3) 3-Amino-Modifier C7 CPG |
| Support | Coupling Efficiency |
| Thymidine (1) | 98.8% |
| Thymidine (2) | 98.7% |
| 3'-PT-Amino-Modifier C3 CPG (1) | 97.4% |
| 3'-PT-Amino-Modifier C3 CPG (2) | 97.8% |
| 3'-PT-Amino-Modifier C6 CPG (1) | 96.9% |
| 3'-PT-Amino-Modifier C3 CPG (2) | 97.8% |
One of our main concerns about the PT-Amino supports was the efficiency of the cleavage reaction from the support by ammonium hydroxide, which led to the lower yield of crude oligonucleotide. Also, we felt it was necessary to compare the cleavage efficiencies of the PT-Amino-Modifier supports under a variety of other, commonly used oligonucleotide deprotection conditions, since a wide range of conditions has had to be developed for the deprotection of modified oligonucleotides. For these supports to become universally accepted, they have to be capable of use with the diverse group of modified bases and dyes we offer that has various sensitivities to basic conditions.
A series of experiments was set up to examine the products from 3'-PT-Amino-Modifier C3 and C6 CPG after deprotection by a variety of popular techniques. As an experimental control, the 3-amino-modified oligonucleotides were compared to a thymidyl 20mer prepared from a standard, ester-linked, thymidine CPG.
Results
The experiments were carried out by synthesizing a thymidyl 20mer on a 15 umole scale on each of the following supports: 3'-PT-Amino-Modifier C3 and C6 CPG, and T-CPG. The initial dimethoxytrityl solutions were collected so that the exact starting scale could be determined, as well as the coupling efficiencies, shown in Table 1.
After synthesis, the supports were dried and carefully weighed into 1 µmole scale aliquots in screw cap vials. Aliquots were then subjected, in duplicate, to the deprotection conditions shown in Table 2. The product was carefully isolated and the yield determined by absorbance at 260 nm. Table 2 shows the results of these deprotection experiments, averaged and normalized to account for differences in actual amount deprotected and for differences in coupling efficiencies. The results are given as ratios to the highest yielding deprotection of the thymidine support, which was the potassium carbonate in methanol method.
The products were analyzed by PAGE for purity. The products all looked exceptional with little n-1 evident for any of the syntheses. No evidence of any partially deprotected species, shown in Figure 2, was observed by mass spectral analyses.
Discussion
The results confirmed that the PT-Amino-Modifier supports do not fully cleave using ammonium hydroxide, although the differences of 10% to 20% seem hardly worth considering given the benefits of having no additional chiral center and the absence of N-acetylated products.2 In general, the various ammonium hydroxide conditions all yielded equal amounts of amino labeled product, which was 80-90% of the yield from the thymidine support, after taking into account the slightly lower coupling efficiencies.
Since neither of the potential partially protected impurities shown in Figure 2 was observed, we can assume that the two phthalimidyl amide bonds cleave well before the linkage to the support. The aromatic amide link apparently does not cleave under these conditions, or some of those side products would be observed in the crude product mix.
We were pleasantly surprised that the UltraFast system using AMA yielded higher results than with the T-CPG. The fact that the longer deprotection gave even better results lends credence to the data. The 10-minute deprotection with AMA is an excellent choice for the PT-Amino-Modifier support. It is convenient to prepare and well suited for todays high throughput requirements.
Unfortunately, but not too surprisingly, UltraMild deprotection conditions with potassium carbonate in methanol yielded considerably less product and should not be considered for use with the PT-Amino-Modifier supports.
In conclusion, we found that with one exception, potassium carbonate in methanol, the PT-Amino-Modifier supports can be deprotected with any of the common deprotection conditions with good results.
We are happy to acknowledge that this work was carried out by our colleagues at TriLink BioTechnologies, Inc. We thank Rick Hogrefe, Paul Imperial and David Colms for expanding our knowledge of the PT-Amino-Modifier supports.
Method |
Temp |
Time |
PT-C3 |
PT-C6 |
T-20 Std |
NH4OH |
RT |
24 hrs |
0.92 |
0.92 |
0.99 |
NH4OH |
RT |
48 hrs |
0.80 |
0.87 |
1.00 |
NH4OH |
55°C |
15 hrs |
0.83 |
0.82 |
0.97 |
NH4OH |
65°C |
4 hrs |
0.72 |
0.79 |
0.96 |
3/1 NH4OH/EtOH |
RT |
48 hrs |
0.87 |
0.91 |
0.95 |
1/1 MeNH2/NH4OH (AMA) |
RT |
2 hrs |
|
|
|
1/1 MeNH2/NH4OH (AMA) |
55°C |
10 min. |
1.04 |
1.11 |
0.90 |
1/1 MeNH2/Nh4OH (AMA) |
55°C |
15 hrs |
1.13 |
1.18 |
0.98 |
0.4 M NaOH in 4/1 MeOH/H2O |
RT |
15 hrs |
0.97 |
1.00 |
0.90 |
K2CO3 in MeOH |
RT |
15 hrs |
0.15 |
0.13 |
1.00 |
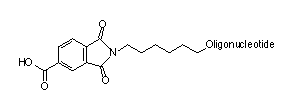 |
| Potential impurity formed by hydrolysis of the amide linkage to the support. |
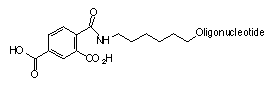 |
| Further hydrolysis would yield this potential di-carboxylic acid impurity. Neither impurity was observed in oligonucleotide products. |
References
(1) C.R. Petrie, M.W. Reed, A.D. Adams, and R.B. Meyer, Jr., Bioconjugate Chemistry, 1992, 3, 85-7.
(2) Glen Report, 2001, 14.1, 5.
Product Information
3'-PT-Amino-Modifier C3 CPG (20-2954)
3'-PT-Amino-Modifier C6 CPG (20-2956)
- Glen Report 15.11: Redmond Red™ and Yakima Yellow™ dyes, and Eclipse™ Non-Fluorescent Quencher
- Glen Report 15.12: Comparison of Deprotection Methods for 3'-PT-Amino-Modifier CPG
- Glen Report 15.13: Pyrrolo-C - A Novel Fluorescent Nucleoside
- Glen Report 15.14: More and Still More Novel Minor Bases
- Glen Report 15.15: A Novel Route to Activated Carboxylate Modified Oligonucleotides

