Glen Report 13.11: C-5 Propynes, Cytofectin and Other Product Updates
C-5 Propynes, Cytofectin GSV
In The Glen Report, 1993, 6(2), 1, we introduced the C-5 Propynyl Pyrimidine derivatives to the research community, under license from Gilead Sciences, Inc. Oligos containing these modifications have proved to be attractive for antisense experiments because of their enhanced binding to their purine partners. At that time, we noted that these properties would also prove to be useful in primers and probes and, in recent years, the majority of uses seem to have been in more routine biological experiments. In The Glen Report, 1996, 9(1), 1, we introduced Cytofectin GSV, a transfection reagent, designed at and licensed by Gilead, to be used to transport antisense oligonucleotides into cells even in the presence of serum. Although optimal for the transfection of antisense oligonucleotides, Cytofectin GSV has also been found to be suitable for plasmid transfection.
In December 1998, Gilead's antisense technology, including the patents covering the C-5 Propynes and Cytofectin GSV, was purchased by Isis Pharmaceuticals, Inc. In March, 2000, Isis terminated our license to continue to manufacture and supply these products, effective July 9, 2000. Glen Research and Isis have been unable to reach a new agreement to allow Glen to continue supplying these compounds in the long term. Further supply of these products is covered by our original contract with Gilead and we may continue to supply inventory existing on July 9, 2000 for a further 12 months, or until the supply is depleted.
We sincerely regret that our association with these products will be ending and, especially, we regret any inconvenience that our customers may incur as a result. Please check our web site for up-to-date information about supplies and for the name and contact information of the responsible person at Ionis.
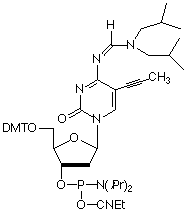
UPDATE: The C-5 Propynes are once again offered with a signed license acknowledgment form, please contact Glen for details.
Product Information
Cytofectin GSV remains discontinued.
pdC-CE Phosphoramidite (10-1014)
The Glen Report 13.1
In this issue, we fortunately have lots of good news to go with the preceding announcement. We have another fine article from Misha Shchepinov from his work in Oxford. This time, he discusses a resurrection of the trityl group with applications in fluorescence, combinatorial chemistry and mass spectroscopy. We would appreciate your input as to which of the products discussed should be made commercially available. In addition, beginning on Page 2, Scott Strobel has kindly updated us on the state of play with Nucleotide Analog Interference Mapping (NAIM) and the newer, more advanced Interference Suppression. And it would not be The Glen Report if there were no new products to discuss, so here we go.
3'-Amino-Modifier C7 CPG 1000
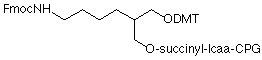
Over the last 18 months, we have been changing our support for minor bases and modifiers to 1000Å CPG. This change has been made to allow longer oligos to be routinely prepared. The analytical information has been amended to include a test to determine where the drop-off point for each specific lot occurs. We think this is more relevant than the pore size of the support in determining the length of oligos that can be made on the support. The drop-off point is now reported for every lot of support. Some products like 3'-Amino-Modifier C7 CPG (20-2957) are in use in the manufacturing of oligos and we will continue to supply the 500Å support. When we add the 1000Å version of such a support, it is given a new catalog number and 3'-Amino-Modifier C7 CPG 1000 (1) is 20-2958.
Product Information
3'-Amino-Modifier C7 CPG 1000 (20-2958)
5-Hydroxy-2'-deoxyCytidine
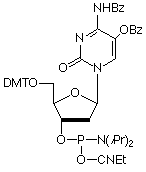
We cannot correctly claim that we are introducing 5-Hydroxy-2'-deoxyCytidine (5-OH-dC) (2) in this issue. Regular readers will, of course, remember that we originally introduced 5-OH-dC in 1998 as a damaged nucleoside analogue ideal for studying free-radically induced DNA damage and enzymatic repair. Unfortunately, the synthesis of the phosphoramidite proved to be "capricious" and we were unable to make any reasonable quantities for quite some time. However, our chemists' perseverance has paid off and we can now offer it again with some confidence.
Product Information
5-OH-dC-CE Phosphoramidite (10-1063)
4-Thio-Uridine
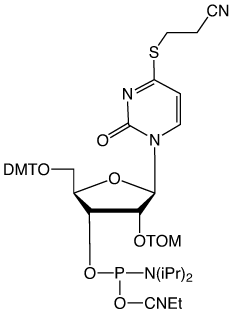
We have been asked frequently in the past to prepare 4-thio-U for RNA synthesis. We were not brave enough to face the difficulties of selecting cleavage and deprotection conditions compatible with this labile base. Now we have made the TOM-protected 4-thio-U phosphoramidite (3) and we have fortunately been able to develop conditions for the cleavage and deprotection steps.
Product Information
4-Thio-U-TOM-CE Phosphoramidite (10-3052)
New Amino-Modifiers



The success of Amino-Modifier C6 dT has prompted us to consider the possibility of supplying the other three standard nucleosides in an amino-modified form. The jury is still out on the two purines but we have been able to produce Amino-Modifier C6 dC as both the phosphoramidite (4) and CPG (5). The side chain is attached at the 5-position of the base and this point of attachment guarantees that there is little or no disruption of hybridization relative to the unmodified dC. We also have taken the opportunity at this time to add the Amino-Modifier C6 dT CPG (6) to our product line.
Product Information
Amino-Modifier C6 dC (10-1019)
3'-Amino-Modifier C6 dC CPG (20-2019)
3'-Amino-Modifier C6 dT CPG (20-2039)
- Glen Report 13.11: C-5 Propynes, Cytofectin and Other Product Updates
- Glen Report 13.12: A Rapid Method for Atomic Mutagenesis of Nucleic Acids
- Glen Report 13.13: Trityl Group in the 3rd Millenium: New Perspectives for Oligonucleotide Chemistry and Beyond
- Glen Report 13.14: Polyplex Synthesizer from Genemachines
- Glen Report 13.15: New Monomers: 2,4-Difluorotoluene, Fluorescein Phosphoramidite and Support, 2',3'-Dideoxynucleosides
- Glen Report 13.16: Product Update - Which 5'-Amino-Modifier?

