Glen Report 25.26: Cyanine Dyes - A Personal Perspective
John B. Randolph, Ph.D.
Glen Research Corporation
With the patents on cyanine dyes having come to a close, this seemed to be a good time to reminisce about the development of this unique class of fluorophores and their rise in popularity. This article is from my perspective having spent many years in the lab of Dr. Alan Waggoner who first developed and commercialized them.
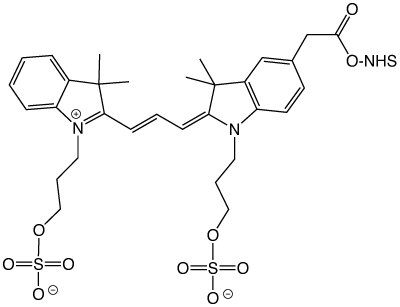
N-Butylsulfonated cyanine NHS Ester
The Waggoner lab was part of an NSF-funded Science and Technology Center, the Center for Light Microscope Imaging and Biotechnology. The Center, deep in the bowels of the Mellon Institute of Carnegie Mellon University, was a mixture of chemists, biologists, computer scientists, and physicists all working together with the goal of developing new technologies and methods to study cell functions and processes. It was a uniquely collaborative environment in which chemists would synthesize dyes and label biomolecules, which in turn were used by biologists to study cellular functions on fluorescence microscopes developed by physicists and automated by computer scientists to collect and store data.
The Waggoner lab focused primarily on the development of new fluorophores and their first major success was a paper on the synthesis and properties of carboxymethylindocyanine dyes as succinimidyl esters.1 This paper was important in a number of respects - first it demonstrated the properties of cyanine dyes quite well:
- the ability to tune their fluorescence emission by changing the number of carbons in the polymethine chain
- their relative insensitivity to solvent polarity and pH
- their high quantum yields of fluorescence and extinction coefficients.
In addition, this paper served to popularize the now ubiquitous "NHS ester" for labelling amines on proteins, oligonucleotides and antibodies. The commonly used reactive moieties at the time - sulfonyl chlorides and isothiocyanates - were often non-specific when labelling and prone to hydrolysis during storage.
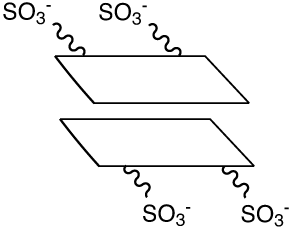
Stacking of dye dimer
However, this early incarnation of what would become Cy3 and Cy5 had issues. To increase the water solubility of the dyes, the starting trimethylindolenine was reacted with butane sulfone to afford an N-butylsulfonated cyanine dye upon condensation, as shown in Figure 1.
Unfortunately, despite their very high water solubility, the butylsulfonated cyanine dyes tended to form dye dimers. Dye dimers (and higher-order H-aggregates) form due to van der Waal and London forces - the result of the hydrophobic and polarizable nature of the dyes. The formation of dye dimers can readily be observed by a simple UV/Vis absorbance spectrum as the appearance of an absorption band that is blue-shifted compared to the monomeric dye - as well as a precipitous drop in fluorescence since the dye dimer is non-fluorescent. In retrospect, the reason is quite obvious - the central aromatic planar ring structures could stack with the charged sulfonates hardly interacting, as shown in Figure 2.
Recognizing this, a new cyanine dye was designed with sulfonates coupled directly to the indolenine rings that would prevent dye-dye interactions by both electrostatic and steric repulsions, as shown in Figure 3. Dr. Ratan Mujumdar succeeded in synthesizing this new set of sulfoindocyanine dyes in what would become one of the most highly cited papers to come out of the Waggoner lab with 689 citations at present.2 The resulting sulfoindocyanine dyes allowed high labelling densities on antibodies and proteins without fluorescence quenching, making them some of the brightest dyes known.
Understanding the potential of these dyes, Alan Waggoner and Lansing Taylor founded Biological Detection Systems (BDS) to commercialize the dyes. Not long afterward, Amersham Biosciences acquired BDS to obtain the rights to the Cyanine dye portfolio of fluorophores. Amersham was subsequently purchased by GE - folding it into their Medical Systems Division that would become GE Healthcare BioSciences.
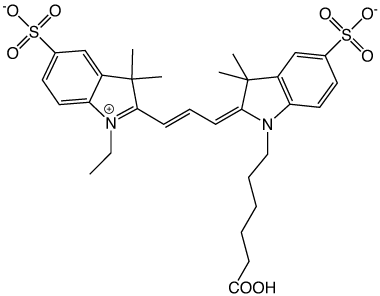
Sulfonates on the indolenine rings prevent dye dimer formation
The new sulfoindocyanine dyes proved extremely useful in a variety of applications due to their high fluorescence brightness and their tendency to show very limited non-specific binding. This allowed the detection and quantification of RNA in single cells by flow cytometry using Fluorescent In Situ Hybridization (FISH)3; the determination of cytoplasm viscosity by ratiometric fluorescence emission of Cy3/Cy5,4 and my own work on the stability, specificity and fluorescence brightness of multiply-labelled fluorescent DNA probes.5
In more recent work, the sulfonated Cy3 and Cy5 dyes were used in the development of STORM - Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy - which harnesses the 'blinking' quality of the cyanine dyes to reconstruct an image down to an amazing 20 nm resolution - far below the diffraction limit.6
While the sulfoindocyanine dyes have wonderful properties, they do have a shortcoming - they cannot be made into stable phosphoramidites which would allow oligonucleotides to be labelled on the DNA synthesizer. As a result, they require a post-synthetic labelling of an amino-modified oligo, subsequent desalting, and typically RP HPLC or PAGE purification.
With the rapid rise of TaqMan PCR analysis and the burgeoning demand for cyanine labelled probes, non-sulfonated versions of the cyanine dyes were introduced by Pharmacia and supplied over the last decade by Amersham, then GE Healthcare BioSciences and, of course, Glen Research under license. These cyanine dyes were compatible with phosphoramidite chemistry which allowed the production of PCR probes directly on the DNA synthesizer, making automation a possibility. As a consequence, TaqMan and FRET probes became more accessible.
Glen Research continues to provide a full portfolio of cyanine dyes with only a change in description. We now describe the dyes as Cyanine 3, Cyanine 5, Cyanine 3.5 and Cyanine 5.5. In addition, we are happy to introduce new supports allowing the facile production of 3' cyanine dye-labelled oligonucleotides.





References
- Southwick, P. L.; Ernst, L. A.; Tauriello, E. W.; Parker, S. R.; Mujumdar, R. B.; Mujumdar, S. R.; Clever, H. A.; Waggoner, A. S., Cyanine dye labelling reagents--carboxymethylindocyanine succinimidyl esters. Cytometry 1990, 11 (3), 418-30.
- Mujumdar, R. B.; Ernst, L. A.; Mujumdar, S. R.; Lewis, C. J.; Waggoner, A. S., Cyanine dye labelling reagents: sulfoindocyanine succinimidyl esters. Bioconjug Chem 1993, 4 (2), 105-11.
- Yu, H.; Ernst, L.; Wagner, M.; Waggoner, A., Sensitive detection of RNAs in single cells by flow cytometry. Nucleic Acids Res 1992, 20 (1), 83-8.
- Luby-Phelps, K.; Mujumdar, S.; Mujumdar, R. B.; Ernst, L. A.; Galbraith, W.; Waggoner, A. S., A novel fluorescence ratiometric method confirms the low solvent viscosity of the cytoplasm. Biophys J 1993, 65 (1), 236-42.
- Randolph, J. B.; Waggoner, A. S., Stability, specificity and fluorescence brightness of multiply-labelled fluorescent DNA probes. Nucleic Acids Res 1997, 25 (14), 2923-2929.
- Rust, M. J.; Bates, M.; Zhuang, X. W., Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nature Methods 2006, 3 (10), 793-795.
Product Information
- Glen Report 25.21: Methylene Blue Phosphoramidite for DNA Labelling
- Glen Report 25.22: New Product - Methylene Blue C3 Phosphoramidite
- Glen Report 25.23: New Product - 5'-Dichloro-Dimethoxy-Fluorescein Phosphoramidite
- Glen Report 25.24: New Products - TEMPO Spin Labels for Click Chemistry and 5-Ethynyl-dU
- Glen Report 25.25: New Product - 5-Formyl-dC III and the Synthesis of Oligonucleotides Containing All Four Epigenetic Nucleosides
- Glen Report 25.26: Cyanine Dyes - A Personal Perspective
- Glen Report 25.27: Technical Brief - Selective Covalent Capture of DNA and RNA Targets with Shielded Covalent Probes Incorporating a Photo-Activated Crosslinker
- Glen Report 25.28: Technical Brief - Glen UnySupport Now Available With Fast Cleavage

