Glen Report 24.16: New Products - Solid, Stable Amino-Modifiers - PDA Amino-Modifiers
Labelling or immobilization of oligonucleotides by conjugation reactions requires the presence of functional groups with unique reactivity towards suitably activated dyes, reporter groups or surfaces. Most commonly, amino-modified oligonucleotides are reacted with active esters of various compounds, thereby forming stable amide conjugates. For the introduction of such amino groups at the 5’ terminus of oligonucleotides, 5’ amino-modifiers are employed. They consist of a phosphoramidite moiety and a suitably protected amino group connected by linkers of various length and structure. Depending on the planned purification, amino linkers with acid or base labile amine protecting groups (for DMT-ON or DMT-Off applications, respectively) can be used. The most popular among the base labile protecting groups is the trifluoroacetyl (TFA) group, which is cleaved by ammonia or methylamine.
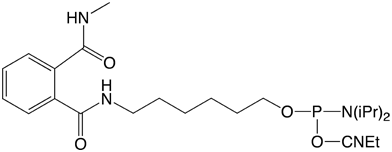
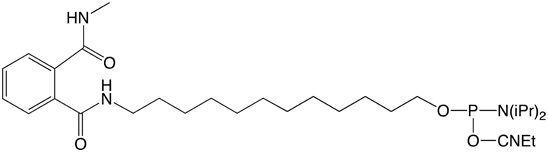
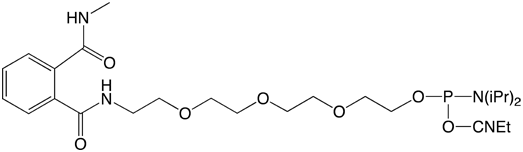
We are now introducing a new set of amino modifiers (Figure 1)1, which are protected by a novel phthalic acid diamide (PDA) protecting group. In contrast to the TFA protected amino modifiers, which are viscous oils, the analogous PDA protected compounds are granular powders. This important property of these compounds allows straightforward handling, storage and aliquoting and leads to a significant increase in stability. For example, at room temperature and under a normal atmosphere, the solid PDA protected amino modifiers are completely stable for at least 7 days. In sharp contrast, the corresponding TFA protected compounds are almost completely degraded under these conditions.
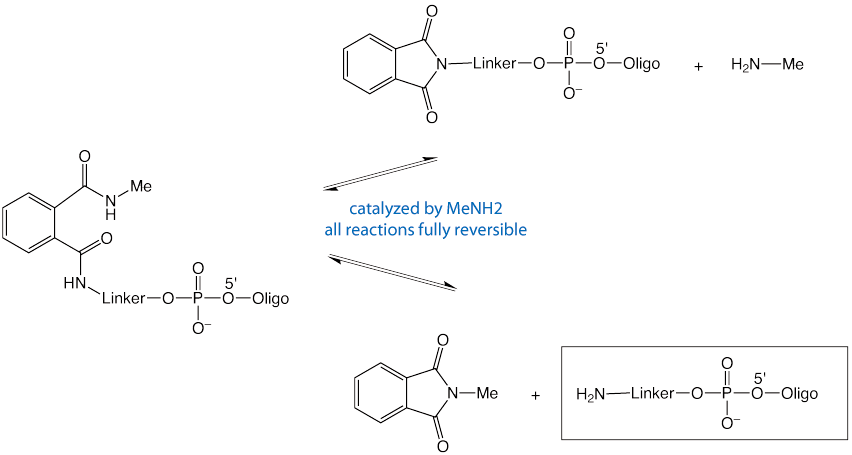
Deprotection with methylamine in gas phase or aqueous solution or AMA leads to fast and complete removal of the PDA protecting group, as shown in Figure 2. However, ammonium hydroxide will not drive the reaction to completion and only partial deprotection occurs.
Glen Research is pleased to offer initially three PDA Amino-Modifiers. Our 5'-Amino-Modifier C6 is our most popular modifier so we offer 5'-Amino-Modifier C6-PDA (1). For longer linkers, we offer the hydrophobic 5'-Amino-Modifier C12-PDA (2) and the hydrophilic 5'-Amino-Modifier-TEG-PDA (3).
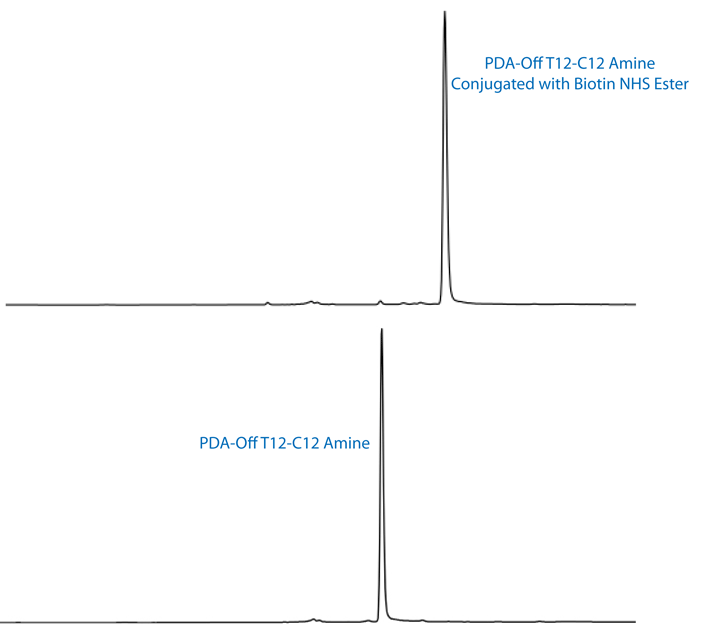
The high conjugation efficiency of amino-modified oligonucleotides prepared using PDA 5'-Amino-Modifiers is demonstrated in Figure 3. A T12 oligonucleotide was modified using 5'-Amino-Modifier C12-PDA. Following deprotection using AMA, very high coupling efficiency was observed. Further, the 5'-amino group was shown to have excellent conjugation potential by reaction with biotin NHS Ester in the normal fashion. Essentially quantitative conjugation efficiency was observed (Figure 3).
We thank Stefan Pitsch for his help in preparing this article. We also thank him for developing a product to address the problems of existing amino-modifiers by providing a new range of products with better stability and handling properties since they are granular powders rather than viscous oils.
1. Developed by Stefan Pitsch and ReseaChem GmbH (S. Berger), Patent pending.
Key Points
- Oligonucleotides containing PDA-amino-modified oligonucleotides must be treated with aqueous methylamine or AMA for complete deprotection.
- If ammonium hydroxide must be used, reaction at 55°C will yield around 80% of the deprotected amino group, even with extended deprotection times.
Product Information
5'-Amino-Modifier C6-PDA (10-1947)
- Glen Report 24.11: Spermine Phosphoramidite: A Potent Modification with Many Applications
- Glen Report 24.12: New Product - S-Bz-Thiol-Modifier C6-dT
- Glen Report 24.13: New Products: AB 3900 Columns Containing 1000Å CPG
- Glen Report 24.14: New Product - Dibenzaocyclooctyl (DBCO) Copper-Free Click Chemistry
- Glen Report 24.15: New Products - 6-HEX and 6-TET Azides
- Glen Report 24.16: New Products - Solid, Stable Amino-Modifiers - PDA Amino-Modifiers
- Glen Report 24.17: New Product - 5'-Stearyl Phosphoramidite - An Alternative Lipophilic Carrier
- Glen Report 24.18: Technical Brief – Compatibility of UltraMild Monomers or Ac-dC with Hydrazine Reagent

