Glen Report 23.27: Technical Brief - Deprotection of HMdU
5-Hydroxymethyl-2’-deoxyUridine (HMdU) is a lesion formed from Thymidine by ionizing radiation or peroxide radicals. HMdU is also interesting because of the current work on the closely related 5-hydroxymethyl-2’-deoxyCytidine monomer (HMdC) which has implications in epigenetic research.
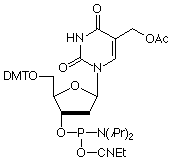
Deprotection of hmdU proceeds with hydrolysis of the acetyl protecting group from the hydroxymethyl group to generate the desired modified nucleoside. However, results generated while working on a different project led us to become concerned that the deprotection of our 5-hydroxymethyl-2’-deoxyUridine (hmdU) monomer (10-1093) (1) might not be as straightforward as we first reported. As part of our policy of continuous improvement, we took a further very close look at the deprotection procedure.

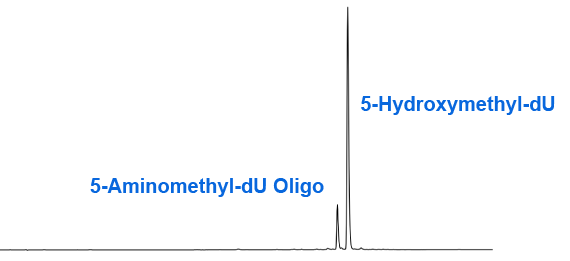
Unfortunately, our suspicions were confirmed when we found that deprotection of an oligonucleotide containing hmdU by heating with ammonium hydroxide for 2 hours at 65°C led to an impurity, as shown in Figure 2. Further analysis confirmed that this impurity was formed by the displacement of the acetate group with ammonia to form the 5-aminomethyl analogue (2). Interestingly, we found that deprotection with ammonium hydroxide at room temperature led to complete hydrolysis and none of the displacement product. The result of deprotection with ammonium hydroxide/methylamine (AMA) at 65 °C was also intriguing in that no displacement reaction was detected (Figure 3). In the case of AMA, the hydrolysis reaction is presumably so fast that the displacement has no chance to occur. Similarly, as expected, deprotection with potassium carbonate in methanol or sodium hydroxide in aqueous methanol gave a pure product identical to Figure 3.

Consequently, we have changed the instructions for the deprotection of oligos containing this modification to:
Synthesize using acetyl-protected dC (10-1015-xx) and deprotect in 30% Ammonium Hydroxide/40% Methylamine 1:1 (AMA) at 65°C for 10 minutes OR synthesize using dmf-protected dG (10-1029-xx) and deprotect in Ammonium Hydroxide for 17 hours at room temperature.
Ordering Information
- Glen Report 23.21: Glen Research Epigenetic Bases Report
- Glen Report 23.22: New Product – 5’-AminoOxy-Modifier 11
- Glen Report 23.23: New Products - BlackBerry® Quencher (BBQ-650®)
- Glen Report 23.24: On-resin Synthesis of Maleimido-oligonucleotides
- Glen Report 23.25: New Product - NPOM-Caged-dT
- Glen Report 23.26: Glen-Pak™ Purification - Then and Now
- Glen Report 23.27: Technical Brief - Deprotection of HMdU
- Glen Report 23.28:Technical Brief - Improved Oligo Synthesis using 2-Amino-dA and CSO Oxidation
- Glen Report 23.29: New Products - Bulk Polystyrene Supports

