Glen Report 23.17: Technical Brief - Chemical Phosphorylation – Considering the Options
As new versions of products are introduced at Glen Research, the older versions may be discontinued or they may have specific attributes that warrant their continuing home in our catalog. In the case of chemical phosphorylation reagents, we have not had the need to discontinue any of our old products that have served us and our customers so well. However, when faced with so many options, customers sometimes need a concise summary of the relative strengths and weaknesses of these products, and we will endeavor to provide that in this Technical Brief.
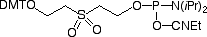
10-1900, 20-2900: Chemical Phosphorylation Reagent and Support
This is our old standby as a chemical phosphorylation reagent (CPR).1 The phosphate is generated by the ß-elimination reaction of a diethylsulfonyl group, as shown in Figure 1. It is an inexpensive and versatile reagent that can be used for 5’-phosphorylation, as well as generating a 3’ phosphate if added to a nucleoside support that will be sacrificed 3’ of the insertion. This can be quite useful if a 2000Å support is required for a very long oligo synthesis but a 3’ phosphate is required. However, a significant drawback of the 10-1900 is that it is not compatible with 5’ DMT-ON purification. In addition, the conditions required to drive the elimination reaction to completion are not the most gentle – in ammonium hydroxide, 17 hours at room temperature or 4 hours at 55 °C. Finally, it is not compatible with standard deprotection conditions in AMA.

10-1901: Chemical Phosphorylation Reagent II
This improved CPR has one very large advantage over the earlier version – it is compatible with DMT-ON purification.2 Figure 2 shows the mechanism of the elimination reaction, with Solid Chemical Phosphorylation Reagent II (10-1902) shown as an example. The first step in the elimination reaction is the abstraction of a proton from the hydroxyl, which occurs under very mild conditions, generating formaldehyde. The rest of the elimination reaction occurs quickly, affording the 5’-phosphate. However, when the DMT group is still protecting the hydroxyl, the elimination reaction is completely inhibited, allowing DMT-ON purification. Upon elution of the purified oligo, treatment for 15 minutes at room temperature in dilute ammonia affords the 5’-phosphorylated oligo.
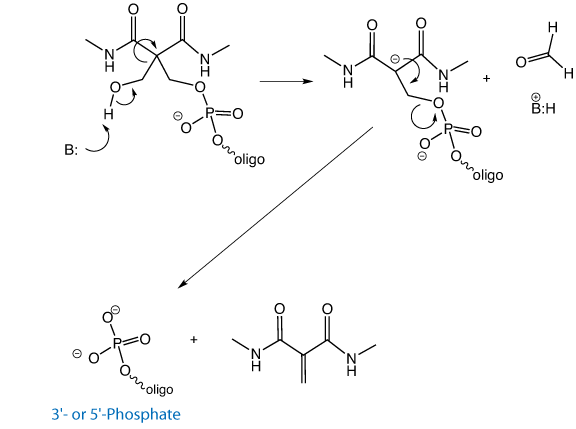
This facile elimination reaction has an additional advantage – when an oligo is synthesized DMT-Off using the Chemical Phosphorylation Reagent II, even the most mild conditions are sufficient to generate the 5’-phosphate. This is very useful when working with RNA or sensitive dyes where deprotection conditions should be as mild as possible.
One additional consideration when using this product is that the synthesis cycle should be modified to remove the capping step after coupling of the phosphoramidite to maintain phosphorylation efficiency.
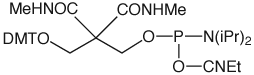
10-1902, 20-2903: Solid Chemical Phosphorylation Reagent II and 3'-CPR II
As the name implies, this phosphoramidite is essentially the solid version of 10-1901. However, its use does not require any changes to the synthesis cycle since it is s to capping and, like 10-1901, it is compatible with DMT-ON purification. In addition, because it is a solid, unlike 10-1900 and 10-1901, it is possible to provide larger pack sizes. As with 10-1901, it allows a method for gentle phosphorylation of RNA if the synthesis is performed DMT-Off.
| DMT-ON Purification | Standard AMA Deprotection | UltraMild Deprotection | DEA Pretreatment | RNA | |
|---|---|---|---|---|---|
| 10-1900 | ✘ | ✘ | ✘ | ✔ | ✔- |
| 10-1901 | ✔ | ✔ | ✔ | ✔ | ✔* |
| 10-1902 | ✔ | ✔ | ✔ | ✔ | ✔* |
| 20-2900 | ✔ | ✘ | ✘ | ✘ | ✔- |
| 20-2903 | ✔ | ✔ | ✔ | ✔ | ✔ |
| *Oligo sythesized DMT-Off (✔-)Indcates an acceptable but not preferred method. |
|||||
Technical Bulletins:
20-2903 versus 20-2900
A common post-synthetic practice performed prior to the cleavage and deprotection step is to treat the support with a solution of 10% diethylamine (DEA) in acetonitrile. This does not cleave the oligo from the support. Rather, it removes the acrylonitrile that is produced from the ß-elimination of the cyanoethyl protecting groups on the phosphodiester backbone. By doing so, alkylation of the N3 position of Thymidine by acrylonitrile is avoided. (An unexplained +53 Da peak in mass spectral analysis of oligos is likely due to alkylation by acrylonitrile.). Given the utility of this procedure, we decided to introduce (in May 2009) Solid CPR II CPG. As with both CPR II and Solid CPR II, the elimination reaction that yields the phosphate does not occur unless there is a free hydroxyl, as shown in Figure 2.
With Solid CPR II CPG, the hydroxyl is not released until the ammonolysis of the succinate linkage has occurred, which means it is perfectly compatible with post-synthesis DEA treatment.
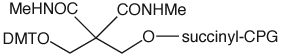
The structures of all of our products for chemical phosphorylation are shown in Figure 3.

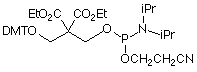
References
- T. Horn, and M. Urdea, Tetrahedron Lett., 1986, 27, 4705.
-
- A. Guzaev, H. Salo, A. Azhayev, and H. Lonnberg, Tetrahedron, 1995, 51, 9375-9384.
- Chemical Phosphorylation Reagent II is covered by US Patent No.: 5,959,090 and European Patent: EP0816368.
Product Information
- Glen Report 23.11: DNA Methylation Revisited
- Glen Report 23.12: New Products Prevent Branching at Secondary Amines using DCI Activator
- Glen Report 23.13: New Products – Click Chemistry Update
- Glen Report 23.14: New Product – Ultrafast Photo Cross-Linker
- Glen Report 23.15: Product Update - Unnatural Base Pairs
- Glen Report 23.16: Technical Brief - Labelling Carboxy-Modifiers with Multiple Reporter Molecules
- Glen Report 23.17: Technical Brief - Chemical Phosphorylation – Considering the Options

