Glen Report 22.14: Saccharin 1-Methylimidazole - An Activator for DNA and RNA Synthesis
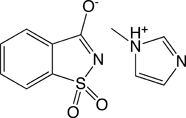
Saccharin 1-methylimidazole (SMI)
Glen Research offers a number of activators for oligonucleotide synthesis and, for some applications, the choice of activator can make a significant difference in overall performance (purity and yields). Our newest offering is saccharin 1-methylimidazole (SMI) (Figure 1). SMI is considered a general-purpose activator for DNA and RNA synthesis. We also currently offer 1H-tetrazole, 5-(ethylthio)-1H-tetrazole (ETT), 5-(benzylthio)-1H-tetrazole (BTT), and 4,5-dicyanoimidazole (DCI).
The classic activator, 1H-tetrazole, performs well in DNA synthesis and can be used in RNA synthesis provided the coupling times (≥12minutes) are adjusted accordingly. Solubility limits, long RNA coupling times, as well as regulatory changes, make 1H-tetrazole a less than ideal general-purpose activator.
In previous studies, it was found that activators that were better proton donors (more acidic) or more nucleophilic, improved the overall rate of reaction.1,2 As ETT and BTT are indeed more acidic than 1H-tetrazole, they are better activators and have been found to reduce the coupling time for RNA synthesis. A risk with acidic activators (pka<4.9) is that they have the potential to detritylate the incoming phosphoramidite during long coupling times. This can lead to dimer phosphoramidite formation, causing a dimer coupling that increases the n+1 peaks in the final product and reduces overall product yields. Dimer addition can be minimized by using a shorter coupling time that still meets the required coupling efficiency.
Activators that are more nucleophilic and less acidic, such as DCI, can reduce the amount of dimer addition and improve the overall yields. This improvement can be significant in the synthesis of long oligos but especially for larger scale oligo synthesis where extended coupling times are less important than overall yield.
The risk with nucleophilic activators is that they can cause branching from secondary amines. Branching is the initiation of a second oligonucleotide sequence from a secondary site on an oligonucleotide, commonly the secondary amines as found on unprotected biotin, N-Ethyl-dC, and N6-methyl-dA. For SMI, branching occurs at a level similar or slightly less than 4,5-DCI. We have found that branching can occur with all activators.
SMI performs very well for DNA and RNA synthesis. (See results in the table below.) We have evaluated SMI in RNA synthesis using TBDMS phosphoramidites using 3 minute, 6 minute, and 12 minute coupling times. Results indicate that a 6 minute coupling time for SMI outperforms ETT at a 6 minute coupling time. Longer coupling times for SMI can realize even further improvements in RNA coupling efficiencies.
| Activator | Type Coupling | CE (%) |
|---|---|---|
| SMI TBDMS RNA | 3 min. | 97.3 |
| SMI TBDMS RNA | 6 min. | 97.8 |
| SMI TBDMS RNA | 12 min. | 97.8 |
| ETT TBDMS RNA | 6 min. | 97.1 |
| SMI DNA | 30 sec. | 99.6 |
| TET DNA | 30 sec. | 99.5 |
The resulting DMT-ON chromatograms using DNA monomers and tetrazole or SMI with standard 30 second couplings are shown in Figure 2. Similarly, DMT-ON chromatograms using TBDMS RNA monomers and ETT or SMI with 6 minute couplings are shown in Figure 3.
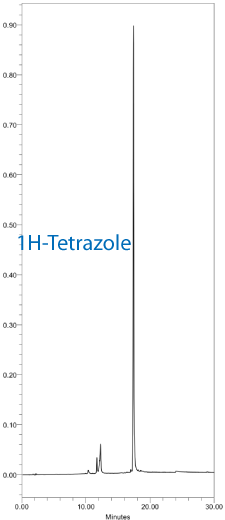
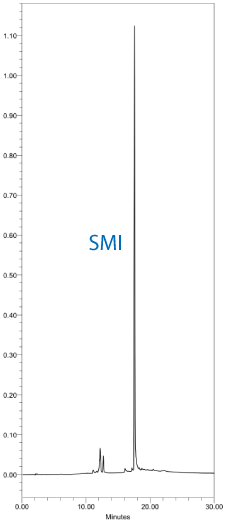
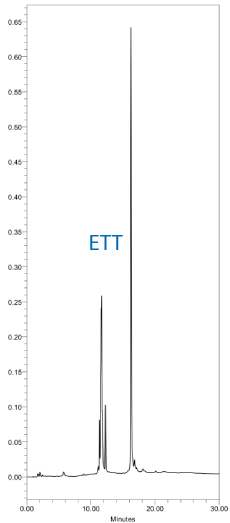

Saccharin 1-methylimidazole is a useful general purpose activator for DNA and RNA synthesis with excellent performance characteristics.
SMI is sold under license from Avecia Biotechnology Inc.
References
- B.H. Dahl, J. Nielsen, and O. Dahl, Nucleic Acids Res., 1987, 15, 1729-43.
- S. Berner, K. Muhlegger, and H. Seliger, Nucleic Acids Res., 1989, 17, 853-64.
Product Information
Saccharin 1-Methylimidazole (SMI) (30-3080, 30-3180) has been discontinued.
- Glen Report 22.11: Simple Oligonucleotide Modification using Click Chemistry
- Glen Report 22.12: The copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC)
- Glen Report 22.13: tC, tCo and tCnitro: Tricyclic Cytosine Probes of DNA and RNA Structure
- Glen Report 22.14: Saccharin 1-Methylimidazole - An Activator for DNA and RNA Synthesis
- Glen Report 22.15: New Product - A dG Amino-Modifier with Improved Hybridization Characteristics
- Glen Report 22.16:; New Products – α-Tocopherol-TEG Phosphoramidite and 3'-Thiol-Modifier 6 S-S CPG
- Glen Report 22.17: New Products - 8-Oxo-G Clamp and 5'-Amino-Modifier TEG CE Phosphoramidites
- Glen Report 22.18: Deprotection – Volume 4 – Alternatives to Ammonium Hydroxide
- Glen Report 22.19: Technical Brief - Depurination
- Glen Report 22.110: Glen-Pak™ Product Update: New Protocols for Desalting and Purification

