Glen Report 22.11: Simple Oligonucleotide Modification using Click Chemistry
For several years, Glen Research has been offering a modest selection of products for click chemistry. However, we have had a few concerns about the technology and we have been reluctant to make a more enthusiastic commitment. Our main concern was strictly practical. In the absence of commercially available and click-tested solutions, the performance of the click reactions was quite variable. In fact, we were very concerned that poor performance in customers' hands would lead to the early demise of this very promising technology. We believe that there are simple solutions to this situation and we are now pleased to give this technology our full attention, as discussed below.
baseclick
Our collaboration with baseclick GmbH has removed our concerns and we now enthusiastically endorse click chemistry. We also expect that it will rapidly become the premiere method for oligonucleotide conjugation. In the accompanying article from baseclick researchers, you will see some representative samples of high efficiency click conjugations. Look to Example 3 to see the results of conjugating an oligonucleotide with biotin at 5 positions. With no purification, the oligonucleotide is essentially 100% pure. The results by conjugating an oligonucleotide at 5 amine positions with biotin NHS ester would be substantially inferior and a difficult purification would be required.
An additional benefit of click chemistry is that conjugations can be done on solid phase on the synthesis column so that excess expensive azide tags can be recovered prior to oligonucleotide deprotection.
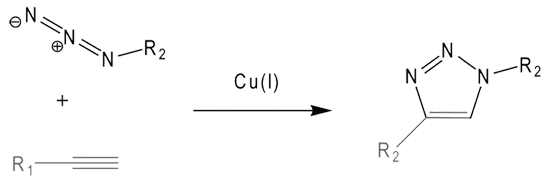
One key ingredient of our collaboration with baseclick is the reagents necessary for successful click reactions. We now offer baseclick grade Cu(I) solutions as well as the Cu(I)-stabilizing ligand required for optimal click condensation reactions.
Single to Triple Click Reactions
More research-oriented examples of baseclick ingenuity can be imagined from the description of click-click and click-click-click conjugations. Using nucleoside alkyne derivatives with and without protecting groups allows up to three separate and independent click reactions to be done, always with the same high efficiency. Try that with a mixture of amino- and thiol-modifiers!
We are delighted to offer the click products described in the following article in collaboration with baseclick GmbH, Tutzing, Germany.
Continued - The Copper(I)-catalyzed Azide-alkyne Cycloaddition (CuAAC)
Product Information
- Glen Report 22.11: Simple Oligonucleotide Modification using Click Chemistry
- Glen Report 22.12: The copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC)
- Glen Report 22.13: tC, tCo and tCnitro: Tricyclic Cytosine Probes of DNA and RNA Structure
- Glen Report 22.14: Saccharin 1-Methylimidazole - An Activator for DNA and RNA Synthesis
- Glen Report 22.15: New Product - A dG Amino-Modifier with Improved Hybridization Characteristics
- Glen Report 22.16:; New Products – α-Tocopherol-TEG Phosphoramidite and 3'-Thiol-Modifier 6 S-S CPG
- Glen Report 22.17: New Products - 8-Oxo-G Clamp and 5'-Amino-Modifier TEG CE Phosphoramidites
- Glen Report 22.18: Deprotection – Volume 4 – Alternatives to Ammonium Hydroxide
- Glen Report 22.19: Technical Brief - Depurination
- Glen Report 22.110: Glen-Pak™ Product Update: New Protocols for Desalting and Purification

