Glen Report 19.29: Technical Brief - About Activators: Now and Tomorrow
1H-Tetrazole
One of the first activators described for phosphoramidite chemistry was 1H-Tetrazole and this has been the mainstay since its introduction for use with DNA synthesis. Other products have been introduced that have advantages over 1H-tetrazole, however none has been truly a universal replacement for 1H-Tetrazole. Nevertheless, the sun may be setting on 1H-Tetrazole as the activator of choice for DNA and RNA synthesis and this note discusses the properties 1H-Tetrazole and its inherent weaknesses as an activator. Several other choices for activator are also described.
The study of the mechanism of 1H-Tetrazole activation by Dahl1, Berner2, as well as others, led to the proposal of a two step reaction of tetrazole with phosphoramidites. First, tetrazole protonates the diisopropylamino group of the phosphoramidite and then displaces diisopropylamine by nucleophilic substitution to form the active species, the tetrazolide intermediate. Subsequent nucleophilic substitution of the tetrazolide with the 5’-hydroxyl of the growing oligonucleotide forms the new phosphotriester linkage. Thus, a better proton donor and/or a better nucleophile to generate the active intermediate should increase the rate of reaction.
Indeed, alternative tetrazole activators such as ethylthiotetrazole and benzyl thiotetrazole have lower pKa, as shown in the Table, and do improve the rate of reaction. Conversely, DCI also improves the reaction rate presumably because it is a better nucleophile.
Some of 1H-Tetrazole’s other properties make it a less than ideal general-purpose activator:
- Limited solubility of 1H-Tetrazole (33.3g/L in acetonitrile) leads to precipitation in transit during the winter months, requiring the solution to be warmed prior to use.
- Similarly, limited solubility leads to precipitation and clogging of the tiny nozzles used in high throughput synthesizers.
- 1H-Tetrazole’s performance in activating sterically hindered phosphoramidites, like RNA monomers, is not optimal.
| Activator | pKa | Solubility in Acetonitrile |
|---|---|---|
| 5-Ethylthio-1H-Tetrazole | 4.3 | 0.75M |
| 5-Benzylthio-1H-Tetrazole | 4.1 | 0.44M |
| 4,5-Dicyanoimidazole | 5.2 | 1.2M |
| Acetic Acid | 4.8 | N/A |
For standard DNA synthesis, none of this is reason enough to supplant 1H-Tetrazole as the activator of choice. However, the classification of the powder, but NOT the solution, as an explosive, may jeopardize reliable supply of 1H-Tetrazole.

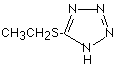
Alternative Activators
5-Ethylthio-1H-tetrazole (ETT) became popular in the 1990s as the preferred activator for RNA synthesis.3-5 ETT is also much more soluble than tetrazole and this attribute certainly has contributed to its more general popularity.
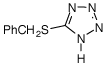
The renewed interest in RNA synthesis due to the growth of siRNA technology has led us to evaluate 5-benzylthio-1H-tetrazole (BTT), which was described several years ago as an ideal activator for RNA synthesis using TOM-protected RNA phosphoramidites6,7 and recently for TBDMS-protected monomers.8 For instance, BTT allows the synthesis of RNA using 2’-TBDMS protected monomers on an AB3900 synthesizer with coupling time around 3 minutes compared to 10-15 minutes with tetrazole. However, although BTT has been widely used for RNA synthesis, it must be remembered that BTT is more acidic than ETT.
A recent study has revealed a major drawback to the acidity of tetrazole-related activators for large scale synthesis.9 This study revealed that tetrazole is sufficiently acidic to deprotect, to a small extent, the trityl group in the monomer solution, leading to a small amount of dimer formation. Coupling of the dimer phosphoramidite leads to the presence of longer oligos (n+1) in the crude product mixture. The conclusion from this study is the more acidic the activator, the higher the risk of double addition and formation of oligos longer than expected. Since these impurities are all trityl-ON at the end of the synthesis, they represent a complication in purification schemes.
An alternative to the tetrazole-based activators is 4,5-dicyanoimidazole (DCI)10 that is less acidic but is a much more nucleophilic activator. DCI is even more soluble in acetonitrile (up to 1.2M solution in acetonitrile). The biggest difference between DCI and tetrazole manifests itself at larger scales that allow the use of a lower excess of monomers relative to tetrazole activators. For example, a 34mer oligoribonucleotide, including 2’-fluoropyrimidine residues, was prepared on a 1 mmole scale with 2 equivalents of monomer using 0.45M tetrazole, 0.45M tetrazole + 0.1M N-methylimidazole (NMI), or 1M DCI as activator. No full-length product was detected with tetrazole activation, while a low yield (13%) of product was observed with the activator containing NMI. With DCI, the full-length product was observed in 54% yield. Our studies with DCI show that 0.25M is the optimal concentration for routine small-scale synthesis (< 15 µmoles), using normal synthesis cycles. We are therefore providing solutions at that concentration but we also offer the raw material so that researchers can prepare more or less concentrated solutions should they desire.
Like ETT, DCI has proved popular for high throughput synthesizers since it also does not tend to crystallize and block the fine outlet nozzles.
Conclusion
For a general purpose activator and for the synthesis of short oligos in small to medium scale, we recommend ETT or BTT. ETT has the added advantage of being more soluble in acetonitrile than 1H-tetrazole (up to 0.75M versus 0.50M solution in acetonitrile). ETT and BTT are more acidic than 1H-Tetrazole and retain its nucleophilic properties. For RNA synthesis, BTT seems to be the best choice as of today. For long oligos and for synthesis at larger scales (>15 umoles), we would suggest using DCI.
Additional activator alternatives also include pyridinium trifluoroacetate, saccharin methylimidazolide (SMI), BTT with methylimidazole, and DCI with methylimidazole. Glen Research is currently reviewing these alternatives to identify the best general multipurpose activator suitable for DNA, RNA, array synthesizers, and large scale synthesis.
Please contact Technical Support at 800-327-GLEN or at support@glenresearch.com, for assistance in selecting the best activator for your application.
References
- B.H. Dahl, J. Nielsen, and O. Dahl, Nucleic Acids Res., 1987, 15, 1729-43.
- S. Berner, K. Muhlegger, and H. Seliger, Nucleic Acids Res., 1989, 17, 853-64.
- B. Sproat, et al., Nucleosides and Nucleotides, 1995, 14, 255-273.
- D. Tsou, A. Hampel, A. Andrus, and R. Vinayak, Nucleosides & Nucleotides, 1995, 14, 1481-1492.
- F. Wincott, et al., Nucleic Acids Res., 1995, 23, 2677-2684.
- X. Wu and S. Pitsch, Nucleic Acids Res., 1998, 26, 4315-23.
- S. Pitsch, et al., Helv Chim Acta, 2001, 84, 3773-3795.
- R. Welz and S. Muller, Tetrahedron Lett., 2002, 43, 795-797.
- A.H. Krotz, et al., Tetrahedron Lett., 1997, 38, 3875-3878.
- C. Vargeese, et al., Nucleic Acids Res., 1998, 26, 1046-1050.
Pruduct Information
- Glen Report 19.21: Glen-Pak™ Cartridges – The Ultimate Purification Cartridges
- Glen Report 19.22: Technical Brief - Procedure for the Synthesis and Deprotection of Synthetic RNA
- Glen Report 19.23: Improved RNA Synthesis Using the Q-Linker for Faster Product Cleavage from Solid-Phase Supports
- Glen Report 19.24: New Lines of Monomers - HT for High Throughput and LC for Low Cost RNA
- Glen Report 19.25: AP-dC is compatible with PCR and is Highly Fluorescent
- Glen Report 19.26: Pyrrolidine CE Phosphoramidite: A New Base Excision Repair Inhibitor
- Glen Report 19.27: New Products - 2,6-Diaminopurine-TOM and Fmoc-Amino-Modifier C6 dT Amidites
- Glen Report 19.28: Versatile New Reagents – Pyrene-dU and Perylene-dU
- Glen Report 19.29: Technical Brief - About Activators: Now and Tomorrow
- Glen Report 19.210: Introducing Thiophosphoramidites

