Glen Report 19.18: Large Scale Synthesis Update
Use of Universal supports to prevent some major impurities in oligo synthesis
With the development of applications requiring large quantities of oligonucleotides, overall yield and synthesis optimization have been investigated in detail. Major impurities have been tracked, analyzed and characterized.
One of these typical impurities can be seen when polyacrylamide gel electrophoresis (PAGE) or capillary electrophoresis is used for analyzing oligos. For example, when looking at a PAGE gel using the UV-shadowing technique,1,2 the band corresponding to the full-length oligonucleotide appears as a large, intensely dark band migrating at the expected position according to its length (n). Bands of various intensities migrating faster than the intended oligonucleotide usually accompany this major band. These bands correspond to failure sequences (n-1, n-2, etc). However, bands migrating much slower than the desired oligonucleotide may also be observed.3 More surprisingly, the somewhat common impurity band has a mobility corresponding to the approximate size expected from a dimer (2n) of the intended oligonucleotide. This impurity is also observed on HPLC, eluting after the expected peak. These impurity products, also known as high molecular weight impurities, have recently been studied and characterized by two groups: one at ISIS4, and one at the University of Bordeaux (France)5.
Both groups made similar discoveries: the bands migrating like dimers of the expected oligonucleotide are in fact branched impurities consisting of two oligonucleotide chains, one linked through the exocyclic amino group of the 3’-terminal nucleoside and the other through the 3’-terminal hydroxyl group via a phosphodiester or a phosphorothioate linkage, depending on the oxidizing process used.
The data presented in these reports indicate that phosphorothioate and phosphodiester oligonucleotides synthesized using A, C, or 5-Me-C derivatized solid supports can contain high molecular weight impurities of the type described. The problem appears more pronounced with 5-Me-2’-OMe-C supports. It is certainly possible to remove these impurities by purification. Or, as pointed out by Cazenave,6 a post-synthetic treatment with neat triethylamine trihydrofluoride would selectively and efficiently cleave any phosphoramidate linkage and convert any N-branched oligonucleotide back to the desired compound.
This treatment would also improve the recovery of oligonucleotides obtained from the N-unprotected phosphoramidite method6,7,8 that despite considerable progress in the discovery of O-selective activators, still produces detectable amounts of N-branched oligonucleotides. However, such an approach definitely reduces the overall synthesis yield. In other words, for a given amount of purified products, one will have to use more phosphoramidite and other reagents. Consequently, the overall cost per purified unit will be higher.
A more efficient approach is to develop chemistries that avoid impurity formation. As presented in the Kurata paper,4 the use of a UNIVERSAL SUPPORT is an excellent approach to circumvent occurrence of these 2n-1 and related impurities.
Glen Research offers several possible solutions and we believe that our Universal Support II (US II) is today the only truly universal support.9 As described in a paper we published in 2005,10 US II performed the best of the group of commercially available universal supports, generating the highest yields of oligonucleotides under the mildest conditions. Only US II is appropriate for the production of DNA oligos, long and short, as well as those requiring mild deprotection. It is also compatible with the synthesis of RNA and siRNA.
Another important advantage of a universal support is that it reduces the need to keep inventory of many pre-loaded supports bearing unnatural nucleosides or labels (LNA, RNA, modified nucleosides). For example, the use of USII polystyrene columns is the best way to synthesize RNA or LNA molecules on an AB 3900 synthesizer. This approach is also useable for synthesis in both orientations, 3’->5’ as well as 5’->3’.
For synthesizing in 96-well (or more) plates, the use of a universal support eliminates the tedious and risk-prone delivery of support loaded with the appropriate base to each well. Plates loaded with universal support can be prepared in advance.
The reagent used for the cleavage/ dephosphorylation step of Universal Support II (anhydrous ammonia in methanol) is commercially available and the procedures described with the product are fully compatible with high throughput synthesis.
This product is available on CPG (bulk, columns for 96 well plates, Expedite or ABI 39x format), on polystyrene (columns for AB 3900 synthesizer) and a new higher functionality polystyrene (US II PS2) (bulk, columns for 96 well plates and ÄKTA oligopilot).
More information on cleavage and deprotection using this support is available on our web site https://www.glenresearch.com/products/universal-supports-and-synthesis-supplies/universal-supports.html
Note: This product is covered by US Patent No.: 6,770,754 and European Patent No.: 1404695.
References
- S.M. Hassur and H.W. Whitlock, Anal Biochem, 1974, 59, 162-164.
- P. Hendry and G. Hannan, Biotechniques, 1996, 20, 258-264.
- A. Andrus, Gel-capillary electrophoresis analysis of oligonucleotides. In Protocols for Oligonucleotide Conjugates, S. Agrawal, Ed. Humana Press: Totowa, NJ, 1993; pp 277-300.
- C. Kurata, et al., Bioorganic & Medicinal Chemistry Letters, 2006, 16, 607.
- C. Cazenave, K. Bathany, and B. Rayner, Oligonucleotides, 2006, 16, 181.
- S.M. Gryaznov and R.L. Letsinger, Tetrahedron Letters, 1992, 33, 4127-4128.
- Y. Hayakawa and M. Kataoka, J Amer Chem Soc, 1998, 120, 12395-12401.
- A. Ohkubo, Y. Ezawa, K. Seio, and M. Sekine, J Amer Chem Soc, 2004, 126, 10884-10896.
- A.V. Azhayev and M.L. Antopolsky, Tetrahedron, 2001, 57, 4977-4986.
- A. Azhayev, et al., GEN 2005, 25 (5). http://www.glenres.com/Reference/25GEN5BPAdvance.pdf
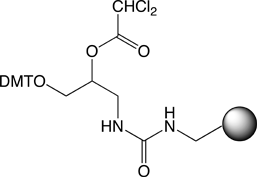
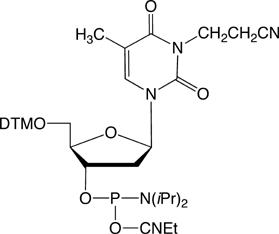
N3-Cyanoethyl-dT
Synthesis of oligonucleotides on a large scale has become increasingly important in a variety of fields, including therapeutics and diagnostics. 2-Cyanoethyl (CE) phosphoramidites are produced on vast scales, are consequently inexpensive, and are by far the reagents of choice for oligonucleotide synthesis. The CE protecting groups are removed from the phosphate backbone by ß-elimination under basic conditions, generating acrylonitrile which is an alkylating agent and known carcinogen. A common side reaction is the alkylation of dT residues by acrylonitrile to form N3-cyanoethyl-dT.1 A recent publication describes2 the use of nitromethane as a scavenger to inhibit the alkylation of dT during deprotection. It is possible to ß-eliminate the CE protecting groups using, for example, diethylamine in acetonitrile while the oligonucleotide is otherwise protected on the solid support. Another option is to deprotect with methylamine which can also act as a scavenger of acrylonitrile but which requires the use of acetyl-protected dC in the oligonucleotide.
Glen Research is pleased to offer N3-cyanoethyl-dT-CE phosphoramidite for use as a standard and to aid in the detection of small quantities of this side reaction in synthetic oligonucleotides that have been produced on a large scale.
References:
- D.C. Capaldi, et al., Org. Process Res. Dev., 2003, 7, 832-838.
- T. Umemoto and T. Wada, Tetrahedron Lett, 2005, 46, 4251-4253.
Reagents for ÄKTA Oligopilot Synthesizer - Update
With the development of applications requiring larger quantities of oligonucleotides, the ÄKTA oligopilot™ (GE Healthcare Life Sciences) has become increasingly popular with our customer base. Also the ÄKTA oligopilot can be used for synthesis under cGMP guidelines, so it is used in many diagnostic and pharmaceutical companies as well as contract manufacturing organizations.
According to GE Healthcare, the batch operation and documentation of this synthesizer are in accordance with the requirements of Good Manufacturing Practice (GMP), Good Laboratory Practice (GLP) and in full compliance with 21 CFR part 11. For additional information on the operating features of the ÄKTA™ oligopilot, please contact GE Healthcare. The high quality of Glen Research products and our production, which is performed under a strict Quality Management System, including full traceability, fits perfectly with this system. Consequently, we have extended our range of products to offer reagents for the ÄKTA oligopilot.
One key feature of the ÄKTA oligopilot is its use of THF-free solutions. In addition, many ÄKTA users prefer the 3% DCA in Toluene deblocking mix since it eliminates the disposal issue of dichloromethane. We have added this deblock solution to our catalog (see Page 12). In addition, we can supply 1.3 ?mol cassettes that will fit on the ÄKTA-10 for smaller scale synthesis, which is helpful for early development synthesis. We can fill these cassettes with all of our available polystyrene supports for 3’-modification, including:
- 3’-PT-Amino-Modifier C6 PS
- 3’-TAMRA-PS
- 3’-Dabcyl-PS
- Universal Support II PS
All of our phosphoramidites in vials designed for ABI 39x/3900 synthesizers will also fit on the slider connection of the ÄKTA oligopilot. And if necessary, we can provide special pricing for packing multi-gram quantities of some products in GL45 bottles, upon request.
Product Information
ÄKTA oligopilot™ columns have been discontinued
- Glen Report 19.11: Modified RNA Phosphoramidites Useful in siRNA Research and Biologically Significant 1-Methyl-adenosine
- Glen Report 19.12: 1-Methyl-Adenine in Nucleic Acids
- Glen Report 19.13: Technical Brief - Precautions During Packaging of Phosphoramidites and Transfer into Alternative Vials
- Glen Report 19.14: Microarrays, Nanotechnology and Beyond
- Glen Report 19.15: 5’-Hexynyl Phosphoramidite – Conjugation with a Click
- Glen Report 19.16: Quenched Autoligation (QUAL) Probes
- Glen Report 19.17: Selenium Derivatization of Nucleic Acids for X-ray Crystal Structure Determination
- Glen Report 19.18: Large Scale Synthesis Update
- Glen Report 19.19: NHS-Carboxy-dT – Expanding the NHS Ester Phosphoramidite Repertoire

