Glen Report 11.22: TOM-Protecting-Group™ - A Major Improvement in RNA Synthesis
Patrick Weiss, Xeragon AG
Xeragon AG and Glen Research Corporation introduce a novel and superior 2'-O-protecting group for automated RNA-synthesis, the TOM-Protecting-Group™ (patents pending):
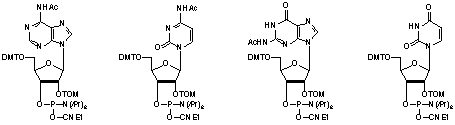
The TOM-Protecting-Group™ is structurally related to the, so far, most successful tBDMS-Group introduced by Ogilvie and Usman in the early Eighties and is fully compatible to the established tBDMS-Chemistry used worldwide. This compatibility has the advantage that one can still use all known and already available modifications. Amidites, shown in Figure 1, containing the TOM-Protecting-Group™ are characterized by the features illustrated in Figures 2-5.
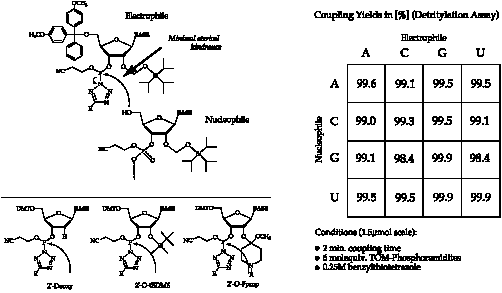
The TOM-Protecting-Group™ solves the problems encountered in automated RNA-synthesis due to the presence of a suitable spacer between the nucleoside and the silyl-group. This minimized steric demand of the TOM-Protecting-Group™ results in excellent coupling yields under DNA-coupling conditions, as illustrated in Figure 2 above.
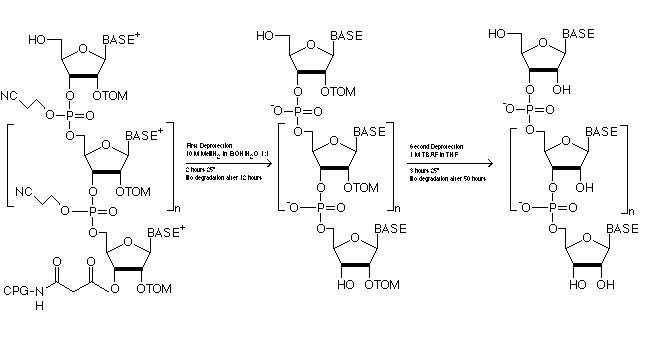
The TOM-protecting group is removed easily and completely under mild conditions leaving the RNA completely intact, as shown in Figure 3. The short deprotection times result in minimized breaking of the strand during deprotection.
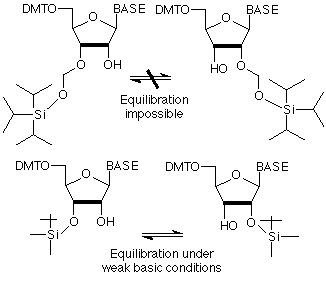
The acetal structure of our TOM-Protecting-Group™ makes it completely stable towards basic and weakly acidic conditions and specifically prevents its migration from 2'-O to 3'-O (which would result in isomeric RNA, containing 2'-5'-phosphodiester linkages).

The synthesis of long and high yielding RNA-Molecules now becomes possible, as illustrated above.
Conclusion
The TOM-Protecting-Group™ is fully compatible with the established RNA-Chemistry, having the advantage of higher coupling yields and shorter coupling times. By making the TOM-Chemistry™ available to a large number of people, we hope to contribute to the field of RNA research and related areas.
References
On the TOM-Protecting-Group™:
Stefan Pitsch, Patrick A. Weiss and Luzi Jenny, "Ribonucleoside-Derivative and Method for Preparing the Same", Swiss Patent Application 01 931/97, August 18, 1997.
Stefan Pitsch, Xiaolin Wu, Patrick A. Weiss and Luzi Jenny, 1998, in Brow, D., Gesteland, R., Krämer, A. and Pyle A. (eds), RNA '98: The Third Annual Meeting of the RNA Society, Program & Abstract, University of Wisconsin, p. 554.
Stefan Pitsch, Patrick A. Weiss, Xiaolin Wu and Luzi Jenny, "Easy and Efficient Chemical Synthesis of Oligoribonucleotides (RNA) from 2'-O-(Triisopropyl)silyloxymethyl-protected Nucleoside Building Blocks", in preparation.
Stefan Pitsch, Patrick A. Weiss, Xiaolin Wu, Damian Ackermann and Thomas Honegger, "Fast and Reliable Automated Synthesis of Oligoribonucleotides Based on Two Novel Orthogonal Protecting Groups for the 2'-O-Position of the Nucleosides", in preparation.
Using the TOM-Protecting-Group™:
Xiaolin Wu and Stefan Pitsch, "Synthesis and Pairing Properties of Oligoribonucleotide Analogues Containing a Metal-Binding Site Attached to b-D-Allofuranosyl Cytosine", 1998, Nucleic Acids Res., 26, 4315-4323.
Martin Huenges, Christian Rölz, Ruth Gschwind, Ralph Peteranderl, Fabian Berglechner, Gerald Richter, Adelbert Bacher, Horst Kessler and Gerd Gemmecker, "Solution Structure of the Antitermination Protein NusB of Escherichia Coli: A Novel All-Helical Fold for an RNA-Binding Protein", 1998, EMBO J., 17, 4092-4100.
Ronald Micura, "Cyclic Oligoribonucleotides (RNA) by Solid-Phase Synthesis", in preparation.
Related Publications:
Pitsch S., "An Efficient Synthesis of Enantiomeric Ribonucleic Acids from D-Glucose", 1997, Helv. Chim. Acta, 80, 2286-2314.
Product Information
- Glen Report 11.21: Advances in RNA Synthesis and Structural Analysis
- Glen Report 11.22: TOM-Protecting-Group™ - A Major Improvement in RNA Synthesis
- Glen Report 11.23: New Columns for Expedite and LV Applications
- Glen Report 11.24: Nucleotide Analogs for Interference Mapping of RNA
- Glen Report 11.25: Alternatives to Expedite Monomers
- Glen Report 11.26: More Novel Monomers

