Glen Report 35-15: Product Review — Rhodamine Dyes
In our previous volume, 34.2, fluorescein dyes were discussed.1 Another popular family of fluorophores is the rhodamine dyes. They are bright dyes that are used in many of the same applications where fluorescein dyes are used including water tracing, paper/textile coloring, food additives (illegally) and biotechnology. The last of these is, of course, the most interesting for our customers.
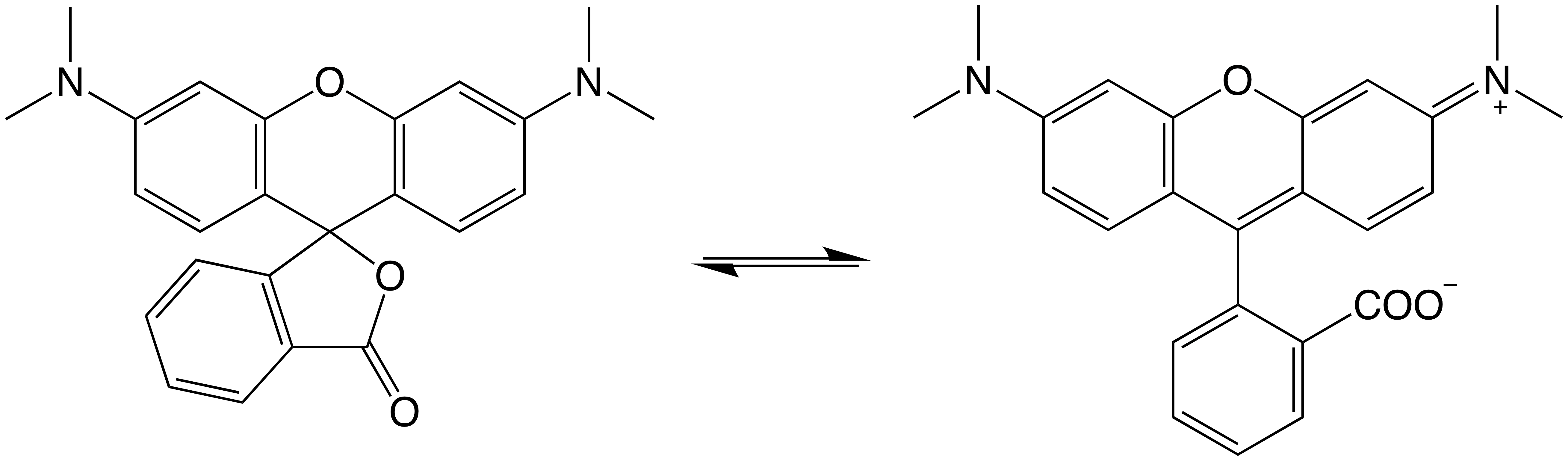
TAMRA is the most common of the rhodamine dyes, at least for oligonucleotide labeling. It is short for TetrA Methyl RhodAmine and consists of rhodamine and four methyl substitutions on the two exocyclic amines (Figure 1). Like most rhodamine dyes, the structure exists as predominantly an equilibrium between the open, zwitterionic fluorescent form (right) and the closed non-fluorescent lactone (left). The small molecule dye has excitation and emission wavelengths of 556 and 580 nm, respectively, and exhibits a pink color. It should be noted that the TAMRA excitation overlaps well with the emission of fluorescein. Although we typically refer to TAMRA as a fluorophore, it is very commonly used as a quencher for fluorescein and its substituted derivatives such as TET and Yakima Yellow® in dual-labeled probes.
Figure 1. TAMRA structure
Glen Research offers three options for TAMRA labeling. There are 3’-TAMRA CPG/PS supports to facilitate 3’-labeling. There is also a TAMRA-dT that can be placed anywhere in the oligonucleotide. Finally, there is an NHS ester that can be attached to any one of our many amino-modifiers via post-synthetic modification. This last approach serves two important purposes. 1. Since there is no 5’-TAMRA phosphoramidite available, the NHS ester is the default choice for 5’-labeling. 2. Due to the incompatibility of TAMRA with standard ammonia-based deprotection methods, the NHS ester approach gives researchers additional deprotection options for more challenging to synthesize sequences.
Figure 2. TAMRA and its derivative products
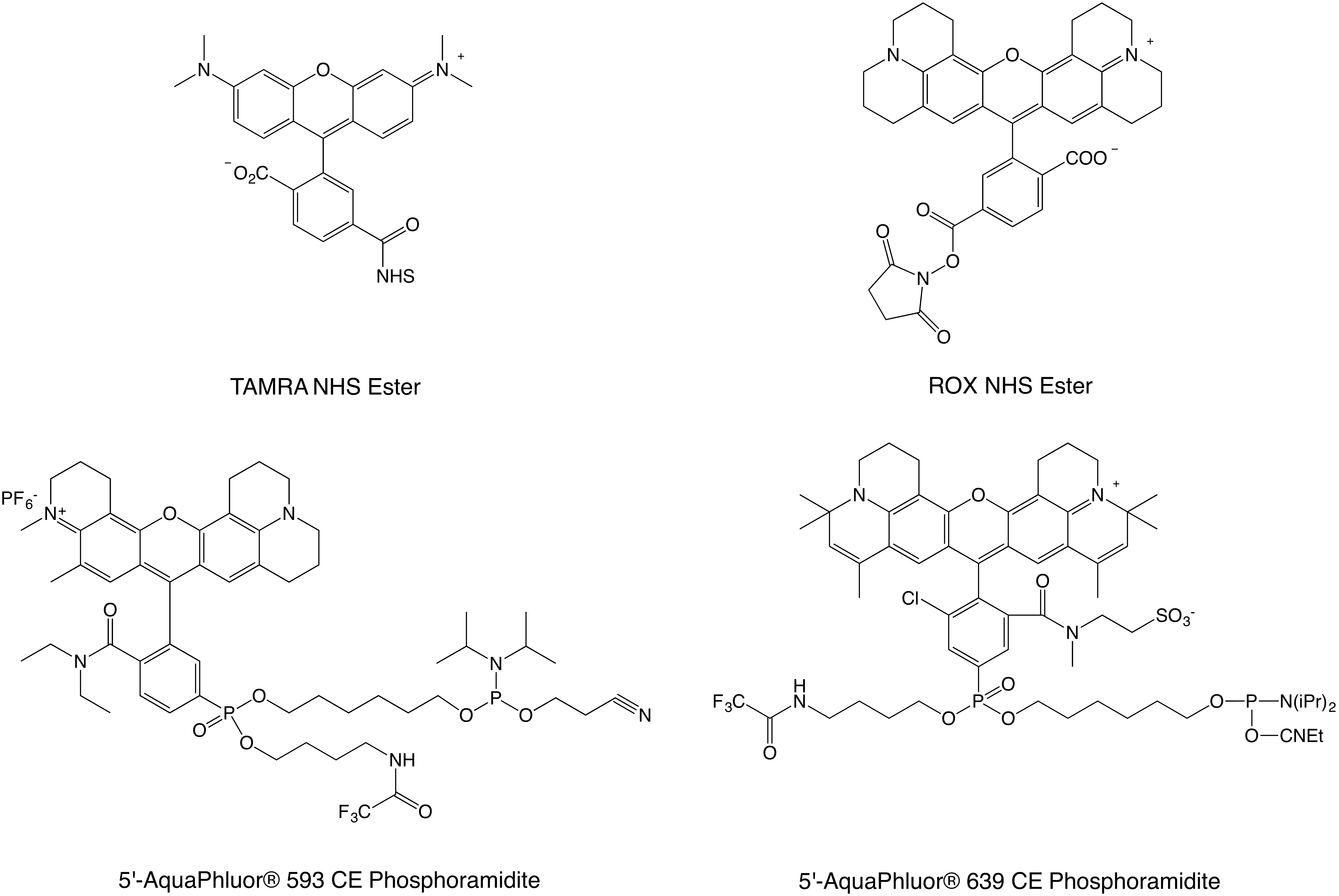
Glen Research also carries other derivatives of rhodamine (Figure 2). Just like our fluorescein family, these derivatives have substitutions on the aromatic rings that red-shift the spectral properties of the fluorophores (Table 1). ROX has additional alkyl rings derived from julolidine and is available as an NHS ester to facilitate post-synthetic conjugation. The core structure of ROX is also available in the form of a 5’-phosphoramidite and CPG as AquaPhluor® 593 (AP593). Further substitution and modification of AP593 gives rise to AquaPhluor® 639 (AP639).
Table 1. Spectral properties of TAMRA and its derivatives
|
Absorbance Maximum (nm) |
Emission Maximum (nm) |
Color |
|
|
TAMRA |
556 |
580 |
Pink |
|
ROX |
588 |
608 |
Magenta |
|
AquaPhluor® 593 |
593 |
613 |
Purple |
|
AquaPhluor® 639 |
639 |
655 |
Blue |
AP593 and AP639 are particularly robust products in the fluorophore offerings. Although they share the same core structure as TAMRA, the AquaPhluors do not share the same deprotection condition susceptibilities. In general, both AMA and ammonium hydroxide are compatible. AP593 essentially allows ROX to be incorporated with no post-synthetic manipulations while AP639 is a rhodamine alternative to Cyanine 5, which is another popular fluorophore that is not very stable under oligonucleotide deprotection conditions.
Rhodamine dyes continue to serve as important members of our fluorophore offerings and complement the other dye families very well.
References
- The Glen Report, 2022, 34.2, 8-9.
Product Information
Rhodamine (TAMRA) Labeling of Oligonucleotides
- Glen Report 35-11: New Product — Azido-Modifier Serinol CPG
- Glen Report 35-12: New Products — NHS Esters
- Glen Report 35-13: New Product — dSpacer-5’-CE Phosphoramidite (Reverse dSpacer)
- Glen Report 35-14: Technical Note — Purification of 5’-Labeled Oligonucleotides Using Glen-Pak DNA Purification Cartridges
- Glen Report 35-15: Product Review — Rhodamine Dyes
- Glen Report 35-16: Technical Note — Sugar Conformations and Modifications
- Glen Report 35-17: Technical Snippets

