Glen Report 35-14: Technical Note — Purification of 5’-Labeled Oligonucleotides Using Glen-Pak DNA Purification Cartridges
Reverse-phase chromatography is a powerful technique that involves the separation of molecules based on their degree of hydrophobicity. It can be used to separate a wide range of molecules, including proteins and nucleic acids. Many purification tools utilize this method such as reverse-phase high-performance liquid chromatography (RP-HPLC) and solid phase extraction (SPE) cartridges. For the former, a hydrophobic reverse-phase column “stationary phase” is used to retain hydrophobic molecules while allowing the hydrophilic molecules to elute quickly through the column by a hydrophilic “mobile phase”. Molecules containing different hydrophobicity levels elute from the column by the gradual increase of organic solvent in the mobile phase. For the latter, the same principles apply, except there is no gradient. Typically, the initial mobile phase allows the molecule of interest to remain bound to the column while unwanted, more polar entities are washed away. The column is then treated with a much more hydrophobic solvent to recover the desired material. In some ways, SPE cartridges are a crude method of performing RP-HPLC for purification of species where there is a very large difference in polarity.
In the oligonucleotide purification space, an SPE cartridge such as our Glen Pak™ cartridge is often all that is required. The 5’-dimethoxytrityl (DMT) group retained on the oligonucleotide during solid phase synthesis can be a significant source of the oligonucleotide’s hydrophobicity, allowing differentiation between desired full length DMT-ON sequences versus undesired shorter DMT-OFF sequences. Upon loading onto the cartridge, the DMT-ON sequence binds to the support while the failure sequences are washed away. As a bonus, the removal of the DMT group can also be performed on the cartridge, allowing full length, DMT-OFF product to be isolated without any additional downstream steps. While the DMT group is an excellent hydrophobic handle for purification on cartridges, in theory, any modification of equivalent or greater hydrophobicity should be able to serve the same purpose.
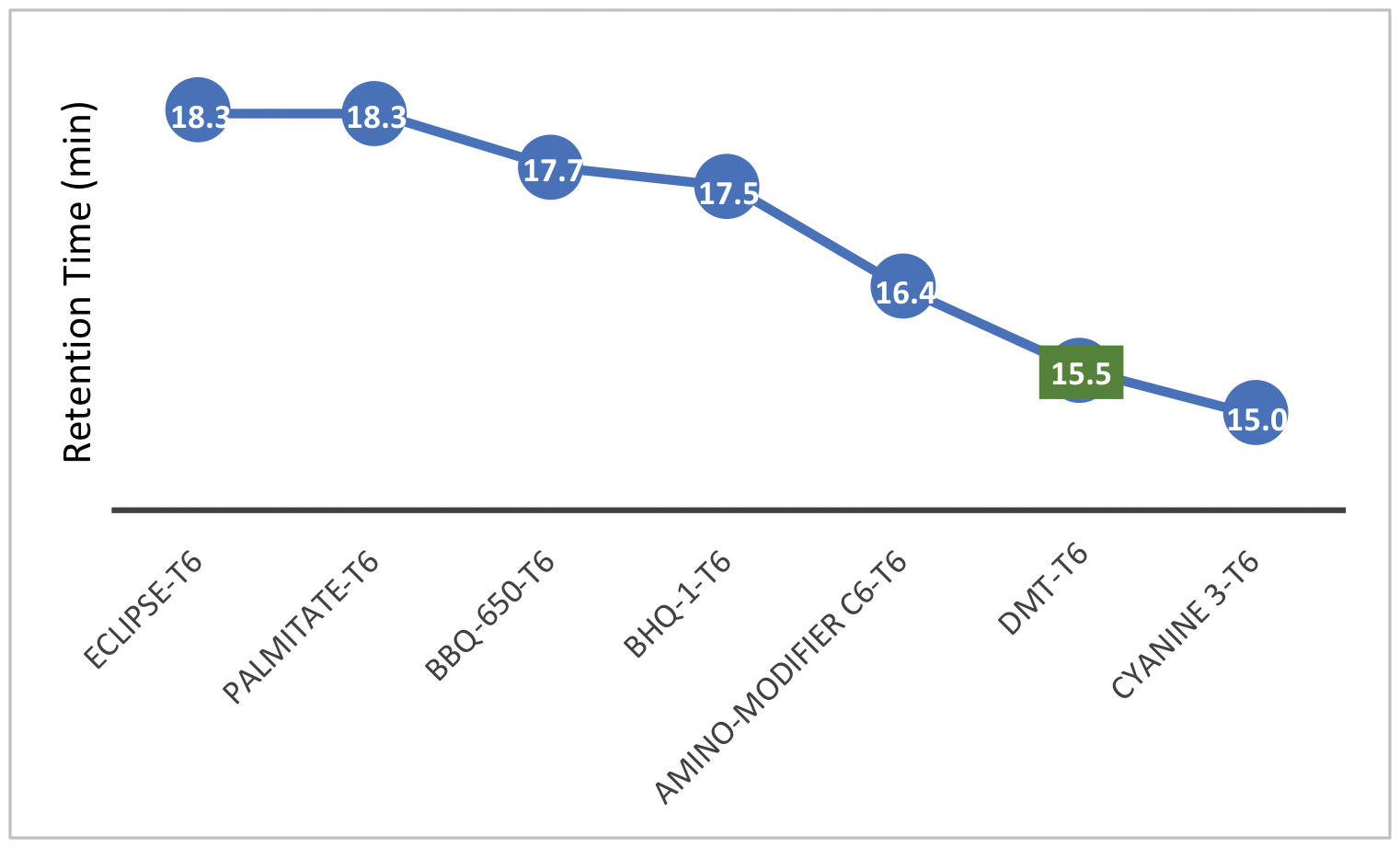
In this article, we aim to assess what other 5’- hydrophobic modifications may be compatible with the Glen-Pak purification process. The retention times (RT) observed during RP-HPLC analysis offer good estimates of hydrophobicity for SPE cartridge purification purposes. Figures 1 and 2 show the retention times of many 5’-labeled T6 sequences, relative to a 5’-DMT-T6 standard. This represents a starting point to determine which modifications are hydrophobic enough to consider purifying with cartridges. RP-HPLC was performed with a gradient of acetonitrile (ACN) in 0.1 M triethylammonium acetate (TEAA). Figure 1 data was generated with a gradient of 3-40 % ACN over 20 minutes while Figure 2 data was generated with a gradient of 3-60 % ACN also over 20 minutes.
Figure 1. 5'-Labeled T6 Oligonucleotide Retention Times (3-40 % ACN gradient)
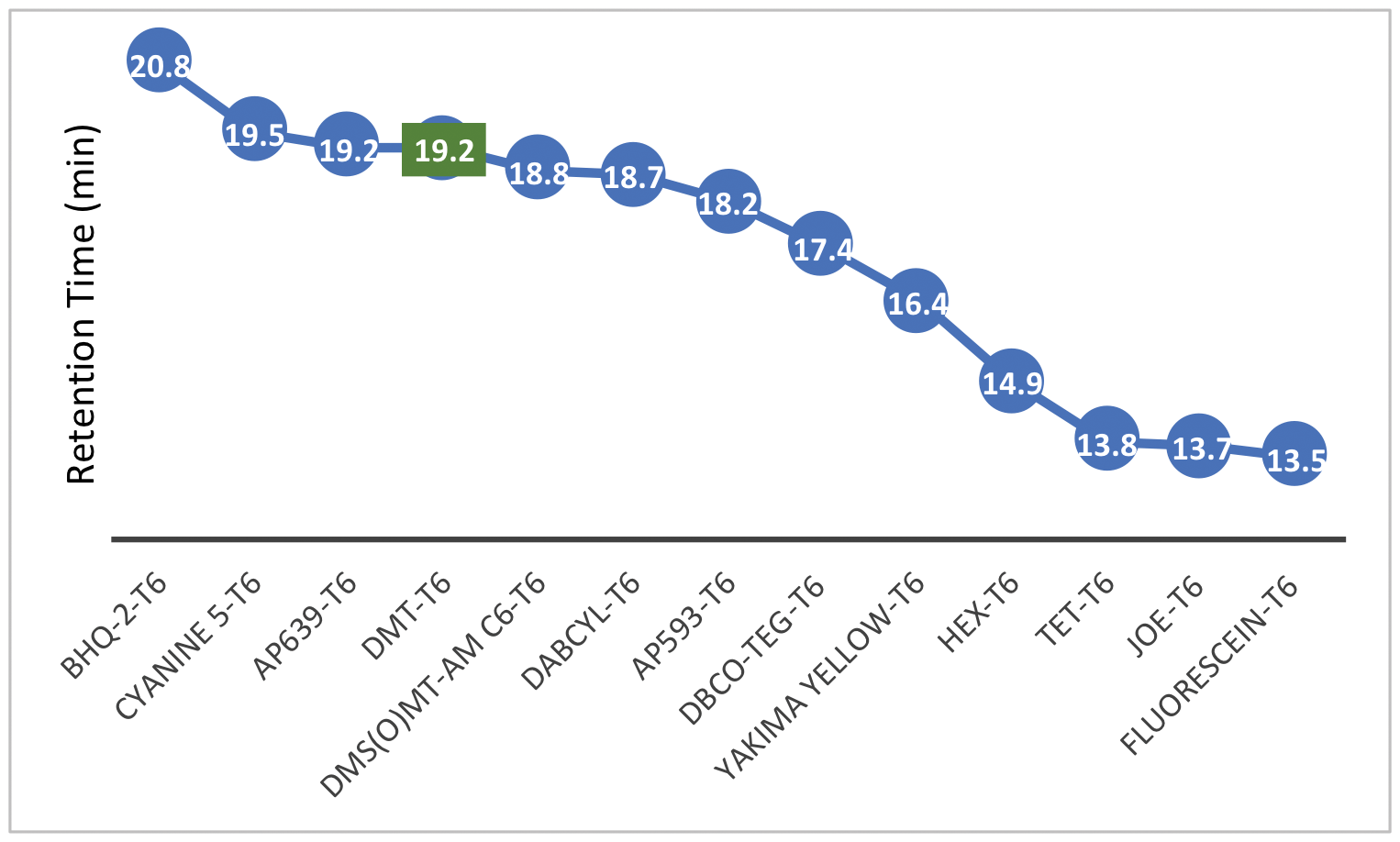
Figure 2. 5'-Labeled T6 Oligonucleotide Retention Times (3-60 % ACN gradient)
Looking at the data, the observed retention times support what we already know. Our User Guide to Glen-Pak™ Purification provides detailed purification methods that cover, among others, the 5’-modifications of DMS(O)MT-Amino-Modifier C6, 5'-Amino-Modifier C6, Cyanine 5, and Dabcyl.1 The RT’s for these 5’- modifications are either later than or comparable to that of the DMT-T6. For instance, Cyanine 5 (Figure 3) has an RT that is slightly later than that of the DMT-T6, and the 5’-Cyanine oligonucleotide is indeed compatible with the Glen-Pak purification method. On the other end of the spectrum, the 5’-Fluorescein, TET, and HEX labels, which we know do not work with this purification process,1 have much earlier RTs compared to that of the DMT-T6. These fluorescein type dyes are simply not very hydrophobic, and our recommendation has always been to use Poly-Pak™ II Cartridges instead, where a somewhat different reverse phase purification is carried out.2
We can now apply this knowledge to other modifications. Based on the RT data and the relevant hydrophobicity to the DMT-T6, we anticipate that the rhodamine dyes AquaPhluor® 593 and 639 (AP593/AP639) should work fine with Glen-Pak cartridges (Figure 1). Quenchers (BHQ-1, BHQ-2, Eclipse, and BBQ-650) also appear to be compatible (Figure 2). With a relatively late RT, Palmitate should work as well. Furthermore, DBCO- and Yakima Yellow-labeled oligonucleotides are less hydrophobic and are probably more challenging for Glen-Pak purification. RP-HPLC purification may be a safer/better alternative for these two modifications. Finally, the JOE modification (Figure 3) yielded a RT consistent with the rest of the fluorescein family and will not be compatible with Glen-Pak cartridges.
Although this is not a comprehensive list of all the labels we offer, we believe the major groups are covered, and the compatibility of other groups can be deduced accordingly. For example, if the Palmitate (Figure 3) is compatible with Glen-Pak purification, other fatty groups like Stearyl and Cholesterol should also work. Likewise, if AP593 (Figure 3) is compatible, then fellow rhodamine TAMRA is likely to work as well.
Figure 3. Representative Phosphoramidite Products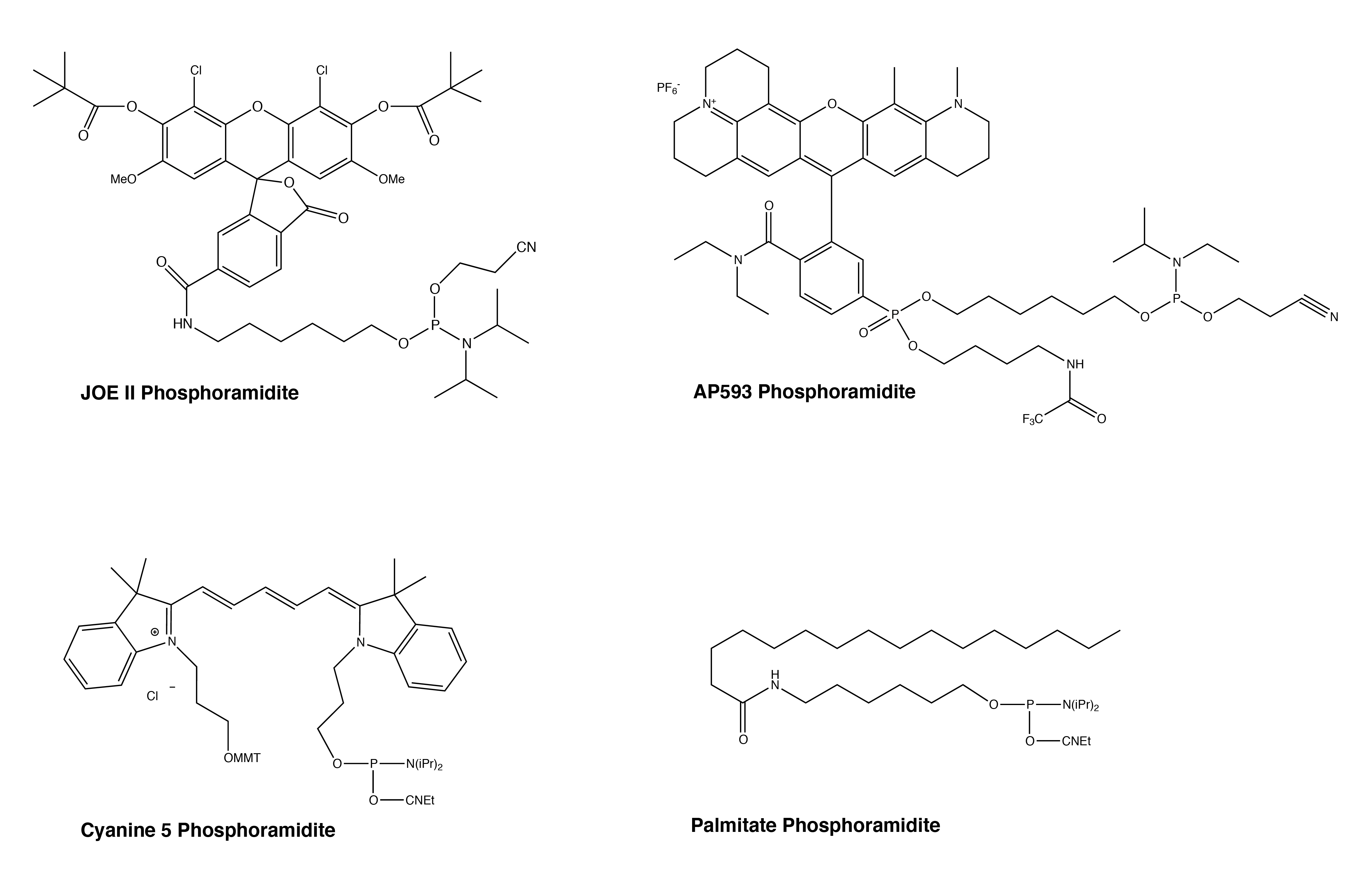
As noted earlier, cartridge purification is a crude but convenient purification method. Even if the purification handles are sufficiently hydrophobic, performance can still vary based on the sequence length as well as sequence content. Longer oligonucleotides will be more hydrophilic, reducing the impact of hydrophobic labels on overall polarity. Also, the presence of other modifications can interfere as in the case of dual-labeled fluorescent probes, such as those used in Fluorescence Resonance Energy Transfer (FRET).3 If the hydrophobicity of the 3’-label is similar to or greater than that of DMT (Figures 1-2), the DMT-OFF failure oligonucleotide sequences will bind to the support and co-purify with the desired product. The same effect applies to internally placed hydrophobic modifications.
In summary, the relative RP-HPLC RT's of 5’-labeled oligonucleotides appear to be reliable predictors of Glen-Pak compatibility, and this should be considered as a planning guide as one decides what purification methods to employ. As always, one may also consider using Glen-Pak purification in tandem with another purification method in order to achieve their ultimate desired purity.
References
- The Glen Report, 2010, 22.1, 15-16.
- The Glen Report, 1999, 12.1, 6-8.
- The Glen Report, 2004, 17.1, 4-6.
Product Information
Glen-Pak™ Reverse Phase Oligonucleotide Purification Products
- Glen Report 35-11: New Product — Azido-Modifier Serinol CPG
- Glen Report 35-12: New Products — NHS Esters
- Glen Report 35-13: New Product — dSpacer-5’-CE Phosphoramidite (Reverse dSpacer)
- Glen Report 35-14: Technical Note — Purification of 5’-Labeled Oligonucleotides Using Glen-Pak DNA Purification Cartridges
- Glen Report 35-15: Product Review — Rhodamine Dyes
- Glen Report 35-16: Technical Note — Sugar Conformations and Modifications
- Glen Report 35-17: Technical Snippets

