Glen Report 34-16: New Product — Palmitate Phosphoramidite
Synthetic oligonucleotides such as antisense oligonucleotides (ASO), aptamers, miRNA, and siRNA have all found their place in therapeutic development. Challenges when using nucleic acids in therapy include targeted delivery, nuclease activity, and membrane permeability. In recent years, scientists have frequently used distinct modifications to improve such biological characteristics of therapeutic oligonucleotides.
It is common to employ sugar and/or backbone modifications, such as locked nucleic acids and phosphorothioate (PS) linkages, to evade nuclease activity and improve biodistribution. ASOs tend to accumulate in the liver, kidney, and spleen after systemic administration. Effective mRNA knockdown in other tissues often requires high dosing.2 Therefore, enhancing the potency of the ASO in other types of tissues through targeted delivery is desirable for certain therapeutic applications. Glen Research has supported these efforts by offering stearyl, cholesterol, α-tocopherol, and GalNAc phosphoramidites (Figure 1).1
 Figure 1. Modifiers that enhance biological characteristics of therapeutic oligonucleotides
Figure 1. Modifiers that enhance biological characteristics of therapeutic oligonucleotides
GalNAc-oligonucleotide conjugates rely on a glycoprotein receptor for cell permeability and directs therapeutic agents to liver cells. Through an alternative mechanism, hydrophobic modifications, like fatty acids and cholesterol, improve cellular uptake and activity in multiple tissues. We are happy to offer our customers a new modification for these efforts: Palmitate Phosphoramidite.
Palmitic acid is a saturated long-chain fatty acid with a 16-carbon backbone. It is naturally found in palm oil and is the most common fatty acid found in the human body. The palmitate phosphoramidite consists of the C16 chain connected to a C6 linker via a traditional amide bond.
Palmitate-Oligonucleotide Conjugates
Palmitate-modified oligonucleotides offer enhanced ASO activity in skeletal and cardiac muscles compared to the unconjugated ASO.3 The greater potency of palmitate-ASOs was attributed to increased plasma circulation and exposure to extrahepatic tissue. The palmitate modification afforded higher affinity to plasma proteins, especially albumin, and lipoproteins (i.e. HDL and LDL). The lipid conjugates also resulted in less excretion of ASO, allowing more exposure to different tissues. A mechanism for palmitate-facilitated ASO circulation to target cells was proposed a few years ago. The palmitate-ASO yielded association with albumin in the bloodstream, which rapidly distributed the ASO across the vascular endothelium to the interstitium of heart and skeletal muscle.3
In another study, a palmitate moiety was employed in a tricyclo-DNA (tcDNA) ASO with and without a phosphorothioate backbone and evaluated for its effectiveness against Duchenne muscular dystrophy (DMD).4 DMD is caused by a genetic mutation in the dmd gene. Therefore, nucleic acids lend an attractive method for treating muscular dystrophy. Many ASOs developed for treating neuromuscular diseases induce splice switching or skipping (Figure 2).
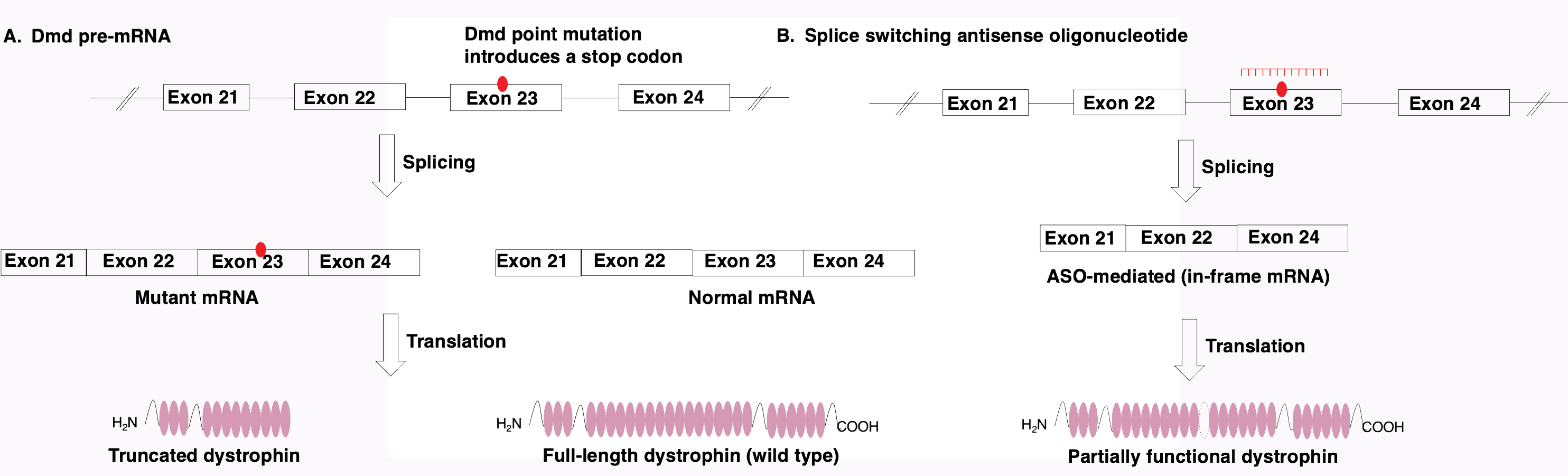
Figure 2. (A) Mechanism of pre-mRNA splicing in dystrophin gene. (B). Mechanism of splice switching antisense oligonucleotides.
Mutations in the dmd gene yield partially or fully inactivated dystrophin protein, leaving muscle tissue more susceptible to degeneration, weakness, and injury. While the loss of dystrophin activity is experienced in all muscle tissue, the disease is life threatening due to degeneration of skeletal and cardiac muscle. Analysis of biodistribution confirmed the palmitate moiety enhanced skeletal muscle accumulation of fully PS-tcDNA-ASOs and fully PO-tcDNA-ASOs 6- and 28-fold, respectively, relative to unconjugated ASO.4 Dystrophin production was also significantly restored in skeletal muscles after treatment with the palmitoyl-ASOs. Perhaps even more impressive, levels of dystrophin increased in neuromuscular tissue, owing to the unique capacity of tcDNA to cross the blood-brain barrier.4
Palmitate conjugates offer enhanced cellular uptake and delivery to extrahepatic tissues. Recent studies using palmitoyl-oligonucleotides suggest promising outcomes as the use of therapeutic oligonucleotides continues to rise.
Recommended Protocols for Palmitate Phosphoramidite
Much like the 5’-stearyl phosphoramidite is not fully soluble in acetonitrile, palmitate phosphoramidite must be dissolved in a mixture of acetonitrile/dichloromethane (1:3). A 6-minute coupling time is recommended. For deprotection and cleavage, no changes are necessary aside from standard methods as required by nucleobases.
References
- The Glen Report, 2012, 24.1, 10.
- S.K. Pandey, et al., J Pharmacol Exp Ther, 2015, 355, 329-40.
- A.E. Chappell, et al., Nucleic Acids Res, 2020, 48, 4382-4395.
- K. Relizani, et al., Nucleic Acids Res, 2022, 50, 17-34.
Product Information
- Glen Report 34-11: Beyond the UV Region — DEACM-dG as a Versatile Tool for Light-Activatable (“Caged”) Oligonucleotides
- Glen Report 34-12: New Product — DEACM Caged-dG
- Glen Report 34-13: Application Note: Phosphorodithioates in Oligonucleotide Therapeutics
- Glen Report 34-14: New Product — Ac-dC-5’-CE Phosphoramidite
- Glen Report 34-15: Purification of RNA Oligonucleotides (DMT-ON) using Glen-Pak DNA Purification Cartridges
- Glen Report 34-16: New Product — Palmitate Phosphoramidite
- Glen Report 34-17: New Product — Universal-CE Phosphoramidite
- Glen Report 34-18: Technical Snippets

