Glen Report 20.26: New Products for Attachment of Oligonucleotides on Gold Surfaces
There has been a lot of development during recent years directed at the application of miniaturization technologies to molecular biology. The ultimate goal is to perform fast molecular tests on microchips. Such microarrays can be fabricated using a variety of technologies, including printing with fine-pointed pins onto glass slides, photolithography with or without pre-made masks, ink-jet printing, or electrochemistry on a microelectrode. These varying technologies allow the manufacturing of microarrays with hundreds, thousands or even millions of specific sequences on solid surfaces that can be glass, silica or gold layered on plastic supports with an area of only 1–2 cm2.1,2
The common factors among all microarrays are:
- Oligonucleotides must be irreversibly attached to the solid surface.
- Hybridization events must be capable of measurement with high accuracy, sensitivity and selectivity, and, if possible in a quantitative way.
Typically, high-density arrays use fluorescence to detect hybridization and such setups are widely commercially available. But there is another technology progressively emerging, which is electrical biosensor detection.3 Osmetech, for example, received FDA clearance in July 2008 for its Warfarin Sensitivity Test and new eSensor® XT-8 platform that uses electrochemical detection and oligos attached to gold surfaces.4
In most cases, electrochemical detection implies that oligos are attached on a gold surface. Typically, oligos are attached on a gold surface using thiol groups forming a self-assembled monolayer (SAM).5 SAMs are molecular layers formed on a surface when it is immersed in a solution containing molecules that specifically interact with this surface. Although different molecules can be immobilized (silanes, carboxylic acids, pyridines, sulphites and thiols) on different surfaces (gold, silver, platinum, copper, mercury and glass), thiols are the most commonly used especially in conjunction with gold surfaces. The stability and organization of the SAMs depend on the forces of attraction between the immobilized molecules, the interaction between terminal groups and their local environment, and the binding force between the surface and the binding group. Oligonucleotide SAMs can be formed directly on the surface when the oligonucleotides contain a pendant thiol group. Or, attachment can be to reactive and previously formed SAMs via EDC. The direct strategy reduces the number of steps required for immobilization and avoids the EDC reaction. However, the indirect strategy (SAMs + EDC) is an alternative when non-saturated monolayers are desired.6,7,8
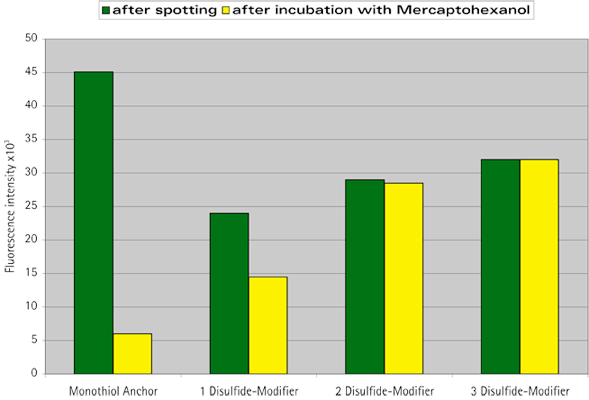
This immobilization of nucleic acids onto substrates is complex and crucial to the performance of the microarray since (i) the capture probe has to form a stable bond to the substrate, (ii) the spacing of the capture probes has to allow specific binding of the target, (iii) nonspecific adsorption of the material to be arrayed has to be prevented. Consequently, chemisorption of thiols on gold (electrodes) is a common and simple procedure to immobilize probes on a surface. The gold–sulfur bond with a binding energy of about 30–45 kcal/mol (cf. at least 100–150 kcal/mol for a covalent bond) is relatively weak in order to anchor a biopolymer onto a surface.9 As reported, mono-functional thiol-terminated oligonucleotides immobilized on a surface are slowly lost at temperatures between 60 and 90 °C and in the presence of buffers with high salt concentration10 and are almost completely displaced from the surface when treated with biological buffer systems containing, e.g., dithiothreitol or mercaptoethanol.11,12
In 2003, Glen Research introduced Dithiol Phosphoramidite (DTPA, 10-1937) as a way to attach more than one thiol residue at the extremity of an oligonucleotide. Considering the success of this product, we have now decided to offer a solid support for the easy introduction of this molecule at the 3’-end of an oligonucleotide. Combining the support and the amidite, it is possible to introduce more than one residue, so multiple dithiol residues, conferring an order of magnitude greater stability to the oligo-gold link, can be easily produced. This has been studied in detail9 and is illustrated in Figure 3, below. This support can be used as any other support and is available on 1000Å CPG. As shown in Figure 2, below, by combining this support and the existing phosphoramidite, it is easy to create a dithiol tail at the 3’-end of an oligonucleotide. The bond between DTPA and the gold surface is formed spontaneously without need of prior reduction of the disulfide bond. That is the beauty of this product. However, it is important that the gold surface is very clean. Piranha solutions (FRIZ Biochem) may be used for cleaning gold electrodes.
We are also pleased to provide a product that completes our roster, 3’-Thiol-Modifier C6 S-S CPG. We have offered Thiol-Modifier C6 S-S Phosphoramidite (10-1936) for many years, but we only offered the C3 S-S support. We are now introducing the C6 S-S CPG support, also on 1000Å CPG, for convenient 3'-thiol labelling of oligonucleotides. The structure is shown in Figure 1, below.

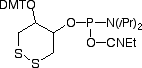
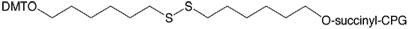
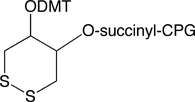
DTPA CPG
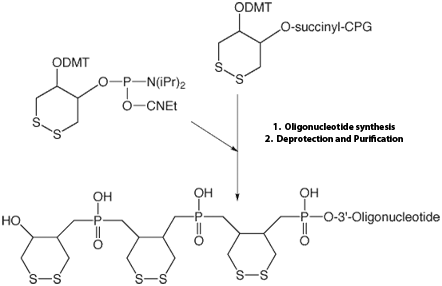
Figure 2: Synthesis of multiple DTPA units at the 3' terminus
References
- M.F. Phillips, et al., Nucleic Acids Res., 2008, 36, e7.
- S.L. Beaucage, Curr Medicinal Chem, 2001, 8, 1213-1244.
- A.A. Gorodetsky, and J.K. Barton, Langmuir, 2006, 22, 7917-22.
- R. Liu, W. Coty, M.W. Reed, and G. Gust, IVDT, 2008, 31.
- R.M. Umek, et al., J Mol Diagn, 2001, 3, 74-84.
- M.R. Lockett, M.F. Phillips, J.L. Jarecki, D. Peelen, and L.M. Smith, Langmuir, 2008, 24, 69-75.
- F. Wei, et al., Nucleic Acids Res., 2008, 36, e65.
- A.G. Frutos, J.M. Brockman, and R.M. Corn, Langmuir, 2000, 16, 2192-2197.
- P. Liepold, et al., Anal Bioanal Chem, 2008, 391, 1759-72.
- Z. Li, R. Jin, C.A. Mirkin, and R.L. Letsinger, Nucleic Acids Res., 2002, 30, 1558-62.
- L.M. Demers, et al., Anal Chem, 2000, 72, 5535-5541.
- R.L. Letsinger, R. Elghanian, G. Viswanadham, and C.A. Mirkin, Bioconjugate Chemistry, 2000, 11, 289-291.
Product Information
3'-Thiol-Modifier 6 S-S CPG (20-2938)
Thiol-Modifier C6 S-S (10-1936)
- Glen Report 20.21: Phosphonoacetate (PACE) Oligonucleotides Introduction
- Glen Report 20.22: Synthesis, Cleavage and Deprotection of PACE Oligonucleotides
- Glen Report 20.23: Novel Reagents for Modification and Labelling
- Glen Report 20.24: Deprotection - Volume 1 - Deprotect to Completion
- Glen Report 20.25: New Universal Support - Glen UnySupport
- Glen Report 20.26: New Products for Attachment of Oligonucleotides on Gold Surfaces
- Glen Report 20.27: 5'-Dichloro-Dimethoxy-Fluorescein (JOE™) Phosphoramidite
- Glen Report 20.28: Site-specific incorporation of functional components into RNA by transcription using unnatural base pair systems

