Glen Report 20.23: Novel Reagents for Modification and Labelling
Reagents for modification and labelling of oligonucleotides have become ever more popular since these reagents allow synthetic oligonucleotides to be tagged at a variety of positions for a multitude of purposes. Our most popular non-nucleosidic phosphoramidites for modification and labelling are based on two structural types as shown in Figure 1: 1,2-diols and 1,3-diols. Figure 1 also shows BiotinTEG Phosphoramidite, an example of a structure based on a 1,2-diol backbone, and Biotin Phosphoramidite, an example of a 1,3-diol based structure. Products based on a 1,2-diol backbone were first described to allow amino-modification1 and biotin labelling2. Of course, Glen Research was in the forefront of these developments, and rapidly offered products of this type for 3’-amino-modification, as well as a family of products for biotin, cholesterol, DNP and fluorescein labelling. Glen Research also introduced a family of products based on the competing 1,3-diol backbone3, including a support for 3’-amino-modification and phosphoramidites and supports for acridine, biotin and fluorescein labelling.
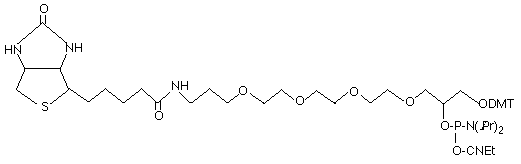
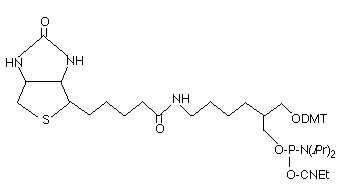
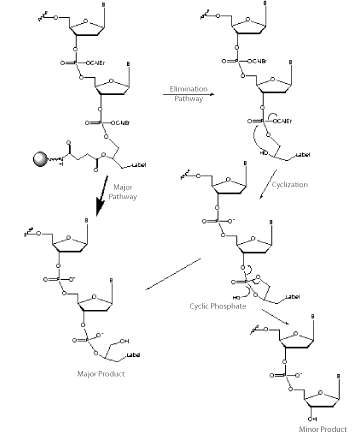
Technically, the 1,2-diol backbone has some drawbacks relative to the 1,3-diol backbone. As shown in Figure 2, the 1,2-diol backbone can participate in a dephosphorylation reaction since the 1,2-diol can form a favored 5-membered cyclic phosphate intermediate. This reaction is competitive with simple hydrolysis of the protecting groups and leads to some loss of label. However, the degree of loss at the 3’ terminus can be limited by the removal of the cyanoethyl protecting group using DBU or diethylamine prior to the cleavage and deprotection steps. Similarly, loss at the 5’ terminus can be eliminated by retaining the DMT group until the oligo is fully deprotected. Fortunately, the elimination reaction is virtually non-existent in the 1,3-diol backbone since the cyclic intermediate would be a 6-membered ring which is not favored for a cyclic phosphate intermediate.
This large variety of products covering the modification and labelling spectrum addresses most of our customers’ needs. However, recently, several of our IVD customers have requested a new backbone based on a 1,3-diol that would overcome any technical or IP issues surrounding our current products. The goal has been to replace the existing 1,3-diol product line with another of equivalent quality and performance with no IP concerns for IVD developers. At the same time, the new biotin structures should be protected to avoid any branching from the biotin molecule itself. Branching from biotin is not very significant for small-scale synthesis but becomes very significant at the larger synthesis scales required by IVD developers.
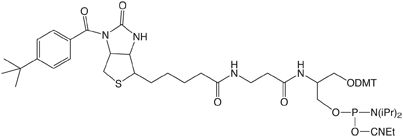
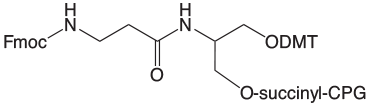
Our response is the line of products, shown in Figure 3, based on the serinol backbone. These products have been developed in close collaboration between Glen Research and Nelson Biotechnologies. At this early stage, we are offering phosphoramidites and supports for Amino-Modification, and Biotin and Fluorescein labelling. These products all exhibit the high purity and performance that is standard for all Glen Research products.
Protected Biotin Serinol Phosphoramidite and CPG are protected with a t-butylbenzoyl group on the biotin ring.4 This group is designed to stop any phosphoramidite reactions at this active position in biotin. This protection avoids branching when using nucleophilic activators like DCI. The protecting group is easily removed during oligonucleotide cleavage and deprotection. The BiotinLC versions are similarly protected and should be useful for the synthesis of highly sensitive biotinylated probes. 6-Fluorescein Serinol Phosphoramidite and CPG are designed to prepare oligonucleotides containing one or several 6-Fluorescein (6-FAM) residues. Amino-Modifier Serinol Phosphoramidite and CPG are used to add amino groups into one or several positions in oligonucleotides. The amino group is protected with Fmoc, which may be removed on the synthesis column prior to solid-phase conjugation to the amino groups, or which may be removed during deprotection for subsequent solution phase conjugation to the amino groups. Further products based on this novel serinol-derived linker may be added in the future.






References
- 1. P.S. Nelson, R. Sherman-Gold, and R. Leon, Nucleic Acids Res., 1989, 17, 7179-86.
- 2. K. Misiura, I. Durrant, M.R. Evans, and M.J. Gait, Nucleic Acids Res., 1990, 18, 4345-4354.
- 3. P.S. Nelson, M. Kent, and S. Muthini, Nucleic Acids Res., 1992, 20, 6253-6259.
- 4. U. Pieles, B.S. Sproat, and G.M. Lamm,Nucleic Acids Res., 1990, 18, 4355-4360.
Product Information
- Glen Report 20.21: Phosphonoacetate (PACE) Oligonucleotides Introduction
- Glen Report 20.22: Synthesis, Cleavage and Deprotection of PACE Oligonucleotides
- Glen Report 20.23: Novel Reagents for Modification and Labelling
- Glen Report 20.24: Deprotection - Volume 1 - Deprotect to Completion
- Glen Report 20.25: New Universal Support - Glen UnySupport
- Glen Report 20.26: New Products for Attachment of Oligonucleotides on Gold Surfaces
- Glen Report 20.27: 5'-Dichloro-Dimethoxy-Fluorescein (JOE™) Phosphoramidite
- Glen Report 20.28: Site-specific incorporation of functional components into RNA by transcription using unnatural base pair systems

