Glen Report 27.23: New Product – Pac-2-Amino-dA
Introduction
As we have noted in the past, the simplest approach to the design of high affinity primers and probes would be to substitute A sites with 2-amino-A, since the 2-amino-A-T base pair is equivalent in strength to the G-T base pair (Figure 1). 2-Amino-A also destabilizes A-G wobble mismatches, thus increasing specificity.
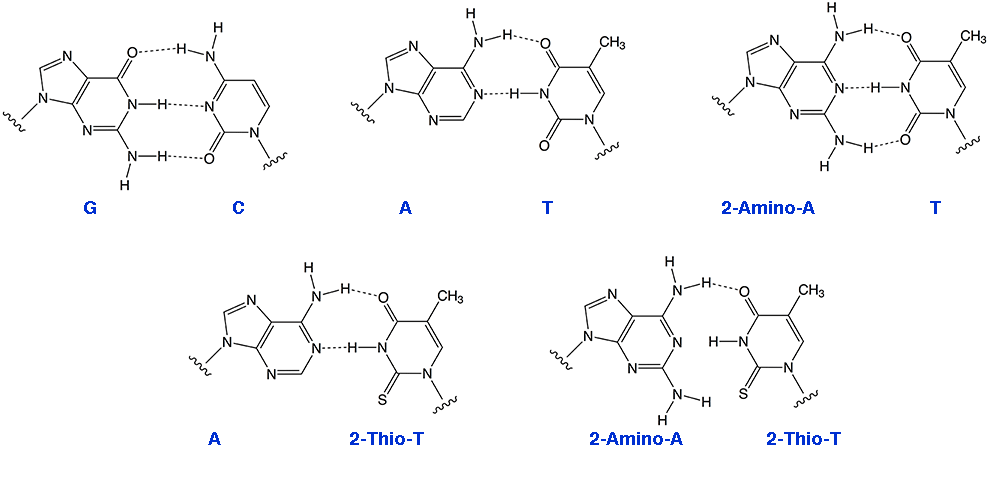
Figure 1: Hydrogen Bonding Patterns for Regular and Modified Bases
In a manner analogous to dA, 2-amino-dA is very susceptible to depurination during the acidic deblocking step of DNA synthesis. From previous development work, we know that acyl protecting groups at N6 seem to favor depurination while formamidine protecting groups seem to suppress depurination during detritylation. In 1998, we introduced the 2-amino-dA monomer (1), which appears to solve all of the earlier problems: deprotection is fast and effective; and it is stabilized to depurination during synthesis. However, the highly nucleophilic character of this bis formamidine protected diaminopurine leads to nucleophilic attack on the C-3’ of the sugar moiety during oxidation with iodine, resulting in strand scission. This problem can be reduced by employing non aqueous oxidation conditions, as described in Glen Report 23.2, page 11. Nevertheless, we concluded that it was time to consider a 2-Amino-dA monomer with optimized protection.
Figure 2: Structures of Monomers Described in this Article
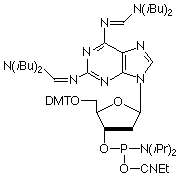
2-Amino-dA (1) |
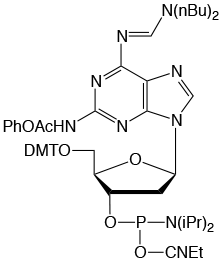
Pac-2-Amino-dA (2) |
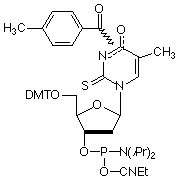
2-Thio-dT (3) |
We defined the following criteria for the “ideal” 2-Amino-dA monomer:
- Stable during oligonucleotide synthesis
- Stable during oxidation (see above)
- Stable during detritylation (depurination)
- Labile towards final deprotection conditions (NH3, AMA, MeNH2)
- The nucleobase protecting groups should be cleavable under mild conditions, e.g., compatible with 2-thio-dT containing sequences.
From previous development work, it is well known that protecting groups at the N2 position are much less labile than identical protecting groups at the N6 position of 2,6-diaminopurine. Therefore, two different protecting groups for these positions were called for. We also rejected the use of two acyl protecting groups since the N6-acyl group would destabilize the glycosidic bond, potentially leading to depurination during detritylation. Consequently, a formamidine protecting group at the N6 position is clearly indicated and dibutylformamidine is a good choice at the N6 position since it suppresses depurination and is easily removed from that position during deprotection. A formamidine at the N2 position causes some strand scission during iodine oxidation, therefore the N2 position should be protected by an acyl group. In order to achieve deprotection of the N2 position under mild conditions, a labile acyl group like Pac or Methoxyacetyl (Mac) should be chosen.
These criteria led us to choose the Pac-Protected 2-Amino-dA and we prepared the phosphoramidite (2) for evaluation.
Synthesis and Deprotection
It was quickly confirmed that Pac-2-Amino-dA coupled as well as regular DNA monomers under identical coupling conditions with no change in coupling time. It was also gratifying to find that iodine oxidation had no effect on the coupling efficiency as confirmed by HPLC analysis of the resulting oligo. So no chain scission was observed using regular iodine oxidation.
Deprotection of oligos containing Pac-2-Amino-dA was analyzed using AMA and ammonium hydroxide. The results are shown below. We found that complete deprotection required about the same time as oligos containing dmf-dG.
| Deprotection Solution | Temperature | Time |
|---|---|---|
| AMA65 °C20 min | ||
| NH4OH | 65 °C | 4 hr |
| NH4OH | 55 °C | 8-17 hr |
| NH4OH | room temp | 24-36 hr |
So far so good, but how would the new product perform in the test we carried out a few years ago with our existing monomer? In that test, we added 2-Amino-dA six times in a mixed base 16mer. The results are shown in Figure 3, where the full length oligo in the crude mixture was only 13% using iodine oxidation but was raised to 60% using non aqueous oxidation with CSO. As noted above, with an electron-donating formamidine protecting group on the N2, the N3 becomes nucleophilic and can attack the C3' causing strand scission and a lower yield of full-length oligo. Also shown in Figure 3 is the chromatogram of the same oligo sequence with six additions of Pac-2-Amino-dA using standard coupling and deprotected with ammonium hydroxide. In this case, the yield of full length oligo was raised to over 95%.

Figure 3: RP HPLC of Crude 16mer Mixed Base OliGo
with six 2-Amino-dA Additions
Duplex Stability Study
With the optimal system in place for the preparation of oligos containing multiple additions of 2-Amino-dA, we carried out an experiment to determine if the increase in melting temperature inherent in the 2-Amino-A-T base pair is cumulative as the substitution of A by 2-Amino-A increases. The following oligos were prepared and their melting behavior examined.
The sequences were:
- Reg AT fwd: 5’-TCT GAA GCT GTT ACT CCG-3’
- Reg AT rev: 5’-CGG AGT AAC AGC TTC AGA-3’
- A 1 Sub: 5’-CGG AGT AAC AGC TTC AGA-3’
- A 2 Sub: 5’-CGG AGT AAC AGC TTC AGA-3’
- A 3 Sub: 5’-CGG AGT AAC AGC TTC AGA-3’
- A 5 Sub: 5’-CGG AGT AAC AGC TTC AGA-3’
The resulting melting plot is shown in Figure 4. The color code is:
| Tm | ||
|---|---|---|
| Blue: | Reg AT bases fwd/rev | 59.9 °C |
| Green: | A 1 Substitution | 60.6 °C |
| Red: | A 2 Substitutions | 62.0 °C |
| Teal: | A 3 Substitutions | 62.9 °C |
| Yellow: | A 5 Substitutions | 65.0 °C |
| where A is 2-Amino-dA. | ||
The resulting melting curves shown in Figure 4 and summarized above indicate that the increase in melting temperature inherent in the 2-Amino-A-T base pair is indeed cumulative. An increase in Tm of 3.0° was observed with three substitutions fairly evenly spaced throughout our test oligo to give an average of 1.0° per insertion relative to the control. When a further two substitutions were made less optimally at adjacent sites in our test oligo, the increase in Tm was 5.1° relative to the control, with the average still at 1.0° per insertion.
Our simple experiment demonstrates that the increase in melting temperature by replacing the A-T base pair with the 2-Amino-A-T base pair is cumulative over five additions in an 18mer.
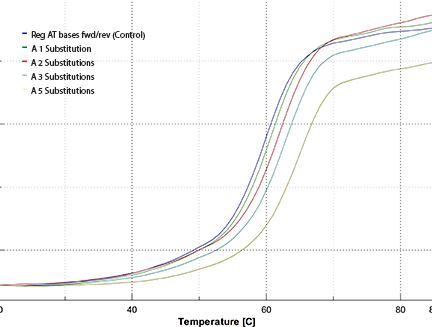
Figure 4: Melting Curves of Oligos Containing 0-5 2-Amino-dA Substitutions
The runs were performed in degassed 0.1 M Tris-HCl, pH 7.4 with the oligos at 1.25 µM
concentration using a Jasco V-530 fitted with a Peltier temperature control unit.
SBC Oligos
Selective Binding Complementary (SBC) oligonucleotides1 are able to form stable, sequence specific hybrids with complementary unmodified DNA or RNA but are unable to form stable hybrids with each other. Oligos in which A has been replaced with 2-amino-A and T with 2-thio-T represent an excellent example of SBC oligos. While 2-amino-A forms a very stable base pair with T containing three hydrogen bonds, the stability of the base pair with 2-thio-T is greatly diminished. The steric interactions between the 2-thio group of thymidine and the 2-amino group of adenine tilt the bases relative to each other yielding a base pair that effectively contains only a single hydrogen bond. However, 2-thio-T base pairs perfectly well with A, as shown in Figure 1. SBC 20mers annealed against a DNA 20mer target exhibited Tm values 10° higher than the corresponding DNA-DNA hybrid, whereas the SBC-SBC hybrid yielded Tm values up to 30° lower.1
2-Thio-dT (3) is best deprotected under mild conditions using ammonium hydroxide at room temperature. Pac-2-amino-dA (2) is also efficiently deprotected in ammonium hydroxide and so is compatible with 2-thio-dT. But is this combination of Pac-2-amino-dA and 2-thio-dT capable of producing SBC oligos? The following experiment with SBC oligos was carried out.
The sequences were:
- SBC fwd: 5’-TCT GAA GCT GTT ACT CCG-3’
- SBC rev: 5’-CGG AGT AAC AGC TTC AGA-3’
- Reg AT fwd: 5’-TCT GAA GCT GTT ACT CCG-3’
- Reg AT rev: 5’-CGG AGT AAC AGC TTC AGA-3’
where A and T are 2-Amino-dA and 2-Thio-dT, respectively.
The oligos were deprotected in ammonium hydroxide at room temperature for 24 hours and purified on Glen-Pak™ cartridges.
Figure 5 shows the melting plot which has been smoothed and normalized to 0.386 AU at 260 nm at 20 °C. The color code is:
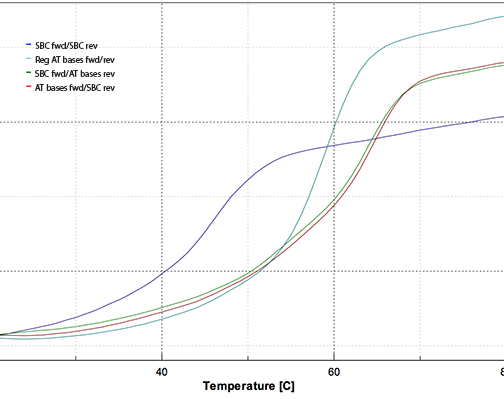
Figure 5: Melting Curves using SBC Oligos and Controls
The runs were performed in degassed 0.1 M Tris-HCl, pH 7.4 with the oligos at 1.25 µM
concentration using a Jasco V-530 fitted with a Peltier temperature control unit.
| Tm | ||
|---|---|---|
| Blue: | SBC fwd/SBC rev | 46.0 °C |
| Teal: | Reg AT bases fwd/rev | 59.0 °C |
| Green: | SBC fwd/AT bases rev | 64.0 °C |
| Red: | AT bases fwd/SBC rev | 64.5 °C |
In our experiment, the SBC-SBC hybrid yielded a Tm 13° lower than the control standard oligo hybrid, while the SBC-regular hybrids had Tm 5° higher than the control oligo. This experiment confirms that our Pac-2-amino-dA monomer allows relatively straightforward production of SBC oligos.
Conclusion
We are happy to offer an alternative monomer for the synthesis of oligos containing 2-Amino-dA. Our goals of designing a monomer that is fully compatible with regular oligo synthesis and deprotection have been achieved, as evidenced by the simple preparation of oligos with multiple 2-Amino-dA residues, as well as the preparation of SBC oligos. We thank Stefan Pitsch of Pitsch Nucleic Acids for his help in the design and production of Pac-2-Amino-dA-CE Phosphoramidite.
Reference
- I.V. Kutyavin, et al., Biochemistry, 1996, 35, 11170-11176.
Product Information
2-Amino-dA-CE Phosphoramidite (10-1085)
- Glen Report 27.21: New Product - 1-Ethynyl-dSpacer CE Phosphoramidite
- Glen Report 27.22: Introducing 2’-OMe-Thiophosphoramidites
- Glen Report 27.23: New Product – Pac-2-Amino-dA
- Glen Report 27.24: New Product - 1-Methyl-Pseudouridine
- Glen Report 27.25: Technical Brief - Dual-labelled Oligos using Click Chemistry
- Glen Report 27.26: New Product - COT Serinol Phosphoramidite

