Oligonucleotides containing multiple labels have practical applications in a number of technologies including PCR, Fluorescence Resonance Energy Transfer (FRET), and Fluorescence In Situ Hybridization (FISH).
Dual-labelled oligo probes are commonly synthesized by phosphoramidite chemistry in a one synthesis-one oligo (OSOO) approach. This synthesis approach can often limit the diversity of fluorophores and quenchers available since they must be compatible with the synthesis and deprotection conditions. Common post-synthesis labelling techniques, including thiol, amine and carboxy labelling, often result in less than robust labelling efficiency for a variety of reasons, such as side reactions with functional groups and deprotection reagents.
Click Chemistry is an alternative post-synthesis labelling technique that is robust, efficient and bioorthogonal. This technique avoids many of the side reactions with naturally occurring functional groups, and may offer better compatibility with standard deprotection techniques.
Glen Research supports two click techniques: copper (I) catalyzed azide-alkyne cycloaddition (CuAAC) (Figure 1); and strained cyclooctyne cycloaddition with dibenzocyclooctyne (DBCO) (Figure 2). Copper-assisted cycloadditions are efficient and, with the THPTA ligand, solution phase conjugation is complete in as little as 15 minutes. The reaction between DBCO and an azide is a simpler and equally efficient conjugation that avoids a copper catalyst.
Figure 1: CuAAC Reaction |
Figure 2: DibenzoCyclooctyl (DBCO) |
 |
 |
DBCO |
In this report, we demonstrate the use of these two click techniques on a single oligo, 5’-DBCO – Oligo – Alkyne-3’, to prepare a dual-labelled probe. The products used are shown in Figure 3. By design, the crude deprotected oligo is ideal for a simple Glen-Pak™ purification since DBCO is hydrophobic and well retained on a Glen-Pak™ cartridge. Failure sequences are eliminated at the load and wash steps, producing a high purity oligo for subsequent labelling.
| Figure 3: Products used for Dual-Labelling | |
 |
|
5’-DBCO-TEG Phosphoramidite |
|
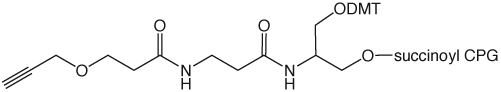 |
|
3’-Alkyne-Modifier Serinol CPG |
|
 |
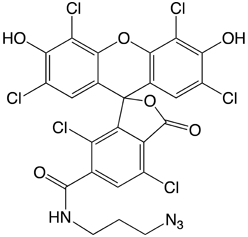 |
6-FAM-TEG Azide |
6-HEX Azide |
 |
|
THPTA |
|
The first conjugation step is to click an azide with DBCO, minus a copper catalyst. The next step clicks a second azide with the 3’- alkyne in the presence of the copper (I) catalyst. This two-step approach produces a dual-labelled oligonucleotide with a profile free of any dye degradation that can occur during deprotection, potentially simplifying HPLC purification. Chromatograms illustrating the procedure are shown in Figure 4.
Figure 4: Progress of Dual-Labelling Oligo Synthesis using RP HPLC |
|
 |
This orthogonal approach allows the incorporation of a diverse set of labels. Notably, one oligo synthesis, when paired with several fluorophore azides, can yield many dual-labelled oligos, resulting in a one synthesis-many oligos (OSMO) approach. This may be especially useful in designing FRET probes or double-labelled FISH probes since many pairs can be evaluated from one oligo synthesis.
Another benefit of this strategy is that the oligo can be further purified by IEX or RP-HPLC prior to labelling resulting in an exceptionally pure oligo. Purification can also be performed after each labelling step, further enhancing oligo purity, which may be important for highly sensitive assays. Additionally, the mild labelling conditions avoid any restriction on labels that could be incompatible with synthesis, deprotection and purification.
Finally, this approach allows tremendous flexibility in oligo design and label selection.