Glen Report 27.12: New Products - Dithiol Serinol Phosphoramidite and 3’- Dithiol Serinol CPG
Gold nanoparticles (AuNPs) have become versatile tools for manufacturing biological sensors based on colorimetric, fluorescent, electrochemical, and other detection techniques.1,2 AuNPs are of particular interest due to their ease of preparation and the diverse options for their functionalization.1,3 For example, aptamers in conjunction with AuNPs have been used for efficient target recognition by using the colorimetric change as the aptamer structure is modified when the target binds.2 For recent reviews on AuNPs, colloidal gold, and other nanomaterials, see Saha et al1 and Wang et al2.
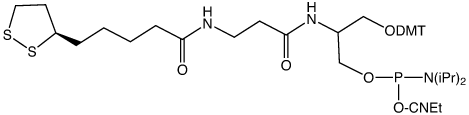

An essential component of the preparation of functionalized AuNPs is the method for attaching ligands to form activated nanoparticles. Due to the strong affinity of thiols and disulfides to gold surfaces and the ready availability of oligonucleotides functionalized with thiol groups at the 3’ or 5’ terminus, thiol-modified oligos have been used extensively for the preparation of oligonucleotide functionalized AuNPs.4
However, Au-S bonds are susceptible to cleavage in the presence of other thiols or at elevated temperatures.5 Consequently, polythiols, such as a dithio-steroid or trebler-thiol-modified oligos, provide a higher level of stability over simple alkylthiol ligands.6,7 The trebler-thiol-modified oligos containing three alkylthiol ligands per molecule exhibited enhanced stability in AuNPs relative to dithiol-labelled oligos.
The introduction of dithiol phosphoramidite (DTPA) offered a straightforward approach to the synthesis of oligo functionalized AuNPs. DTPA exhibited a combination of ease of oligo synthesis with the ability to add multiple dithiol-ligands and ultimately generate very stable AuNPs. Indeed, multiply DTPA-modified oligos provided AuNPs with stability equivalent to or greater than those prepared with trebler-thiol-modified oligos.
DTPA has proved to be a popular product that Glen Research began offering in 2003.5 However, effective January 1, 2015, Glen Research has discontinued DTPA and it is now available directly from FRIZ Biochem in Germany.
Our research into multiply thiol-containing structures led us to the readily available lipoic acid (thioctic acid). Lipoic acid has already been used extensively for labelling gold nanoparticles and surfaces.8-11 Combining lipoic acid and our patented serinol backbone, Glen Research is pleased to offer our Dithiol Serinol Phosphoramidite (1) and the related 3’-Dithiol Serinol CPG (2). This unique architecture incorporates a linker that moves the bulky dithiol away from the phosphate backbone. The longer spacer arm of Dithiol Serinol also allows multiple consecutive incorporations of the modifier without the need for intermediate spacer phosphoramidite additions to achieve optimal stepwise coupling efficiency.
Use of Dithiol Serinol
Our initial experiments have confirmed that Dithiol Serinol Phosphoramidite can be easily incorporated into oligonucleotides using standard procedures of synthesis and deprotection. We have confirmed that Dithiol Serinol Phosphoramidite can be added several times consecutively in high yield without the need for a spacer between each addition.
Dithiol Serinol has been used by several groups experienced in work with AuNPs. Our collaborators have confirmed the ease of use and purification of multiply dithiol-labelled oligos. Their feedback also indicates that oligos produced using this new modifier give coverage of gold surfaces similar to equivalent multiply thiol-modified oligos.
References
- K. Saha, S.S. Agasti, C. Kim, X. Li, and V.M. Rotello, Chemical Reviews, 2012, 112, 2739-2779.
- H. Wang, R. Yang, L. Yang, and W. Tan, ACS Nano, 2009, 3, 2451-2460.
- M. Giersig, and P. Mulvaney, Langmuir, 1993, 9, 3408-3413.
- C.A. Mirkin, R.L. Letsinger, R.C. Mucic, and J.J. Storhoff, Nature, 1996, 382, 607-9.
- http://www.glenresearch.com//GlenReports/GR16-12.html
- R.L. Letsinger, R. Elghanian, G. Viswanadham, and C.A. Mirkin, Bioconjugate Chemistry, 2000, 11, 289-291.
- Z. Li, R. Jin, C.A. Mirkin, and R.L. Letsinger, Nucleic Acids Res, 2002, 30, 1558-62.
- J.A. Dougan, C. Karlsson, W.E. Smith, and D. Graham, Nucleic Acids Research, 2007, 35, 3668-3675.
- J. Sharma, et al., J Am Chem Soc, 2008, 130, 7820-1.
- J.A. Dougan, A.K. Reid, and D. Graham, Tetrahedron Letters, 2010, 51, 5787-5790.
- S. Perez-Rentero, S. Grijalvo, G. Penuelas, C. Fabrega, and R. Eritja, Molecules, 2014, 19, 10495-523.
Ordering Information
Dithiol Serinol Phosphoramidite (10-1991)
3'-Dithiol Serinol CPG (20-2991)
- Glen Report 27.11: Reversible Photo-Switching of DNA Function with Azobenzene-Tethered DNA
- Glen Report 27.12: New Products - Dithiol Serinol Phosphoramidite and 3’- Dithiol Serinol CPG
- Glen Report 27.13: Technical Brief - APA: An Alternative to AMA Deprotection
- Glen Report 27.14: Photocleavable Biotin Linker for Use in SOMAscan™
- Glen Report 27.15: PC Modifiers
- Glen Report 27.16: Technical Brief - Capping and Trityl-Protected Amino-Modifiers
- Glen Report 27.17: Technical Brief - DBCO-dT - An Unusual Case of Iodine Sensitivity

