Glen Report 26.14: Technical Brief - AMA – Ubiquitous Reagent for Oligo Deprotection
Introduction
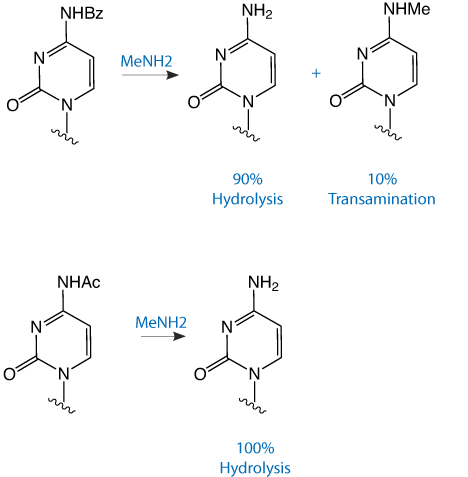
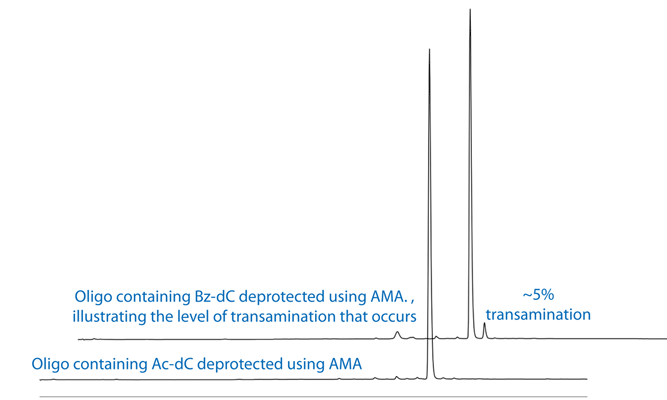
In December 1993 in Glen Report 6.2, we introduced our customers to UltraFAST oligonucleotide deprotection using AMA (Ammonium Hydroxide/40% aqueous MethylAmine 1:1 v/v), originally developed by MP Reddy and his group at Beckman Instruments.1, 2 The presence of methylamine allows the deprotection of the exocyclic base protecting groups, including iBu in dG, in 10 minutes at 65°C. However, if AMA is used with benzoyl protected dC, hydrolysis to the desired dC is accompanied by transamination to form N4-Me-dC at a level of around 5%. With the use of acetyl protected dC, hydrolysis is almost instantaneous and no N-Me-dC is observed. The transamination reaction with Bz-dC is illustrated in Figure 1. HPLC analysis of oligonucleotides containing Bz-dC and Ac-dC and deprotected with AMA is shown in Figure 2.
As we noted in the article, the reduction in time of the deprotection step from many hours to a matter of minutes should relieve the production bottleneck when trying to manufacture large numbers of oligos each day. Our prediction was indeed correct and AMA or variations thereof (aqueous methylamine, methylamine gas, or propylamine in regions where methylamine is controlled) has become the principal method for deprotection in the high throughput synthesis of unmodified DNA oligos.
In this article, we will focus on the advantages that AMA offers in a variety of circumstances in oligonucleotide synthesis and modification. It should be stressed that the use of acetyl-protected C is mandatory in each application we describe.
RNA Synthesis
At the time of introduction of AMA, oligoribonucleotide synthesis was something of an art and it was regularly noted that the presence of the 2’-OH made RNA synthesis an order of magnitude more complex than DNA synthesis. In an early paper3 on RNA synthesis, Reddy compared the use of AMA and aqueous methylamine with the most popular reagent for RNA deprotection at the time, ammonium hydroxide/ethanol 3:1 in this example. In addition to faster cleavage and deprotection, the authors also noted a significantly higher yield of full length product.3
After many years of development work in labs all over the world and much work in our own lab to confirm – Glen Research now recommends AMA as the optimal reagent for RNA deprotection. We offer RNA monomers protected with TBDMS or TOM4 protecting groups at the 2’-OH and both types can be deprotected with AMA. We suggest carrying out the synthesis DMT-ON to allow the oligo to be purified with an RNA Glen-Pak. The simple procedure is outlined below:
- Synthesize oligos DMT-ON.
- Deprotect with AMA for 10 minutes at 65°C.
- Evaporate to dryness.
- Remove 2’-silyl protecting groups using TEA.3HF.
- Dilute with RNA Quenching Buffer.
- Purify using RNA Glen-Pak cartridges.
- More details of this procedure were published in Glen Report 19.2.
TFA Protected Amino-Modifiers
One of the most perplexing issues that arose in the early days of amino-modifiers protected with a trifluoroacetyl (TFA) protecting group was partial inactivation of the amine after deprotection with ammonium hydroxide. We were able to show that the amine, once deprotected, could be alkylated by acrylonitrile formed by elimination of the phosphate cyanoethyl protecting groups. More interestingly, the alkylamine formed was capable of deprotecting acyl protecting groups on adjacent bases to yield a varying percentage of inactive acyl capped amines. This situation is described in detail in the following link:
These competing side reactions are minimized, though not eliminated, by using AMA for deprotection since the desired oligonucleotide alkylamine is simply swamped by the vastly higher concentration of methylamine.
3’-PT-Amino-Modifiers
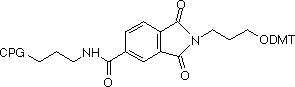
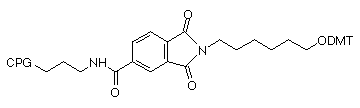
In these supports, the amino group that is destined to be the 3’-amino-modification is incorporated into a phthaloyl (PT) group and is fully protected throughout the synthesis procedure. The amino group is then fully hydrolyzed from the phthaloyl moiety by standard cleavage/deprotection with AMA at 65°C for 10 minutes. There are no side reactions and only pure 3’-alkylamine is released into solution. The structures of the 3'-PT-Amino supports are shown in Figure 3. Also shown in Figure 3 are the potential impurities during hydrolysis of the 3'-PT-Amino-Modifier C6, none of which have been observed with AMA deprotection.

In contrast, other 3’-amino-modifiers, where the amine is protected with the Fmoc group, have several drawbacks in comparison to the 3’-PT-Amino-Modifiers, as described in Table 1.
We can conclude that, used with AMA, the 3’-PT-Amino supports are undoubtedly the best option for the preparation of 3’-amino-modified oligonucleotides. More details of the 3’-PT-Amino-Modifiers were provided in Glen Report 15.1.
| Fmoc Protected | PT Protected |
|---|---|
| Loss of protection possible during synthesis and then the desired amine is irreversibly capped as acetate | No capped amine |
| Chiral center in the branched structure | No branch |
| Potential elimination of linker to 3’-OH during deprotection | No elimination |
5’-PDA-Amino-Modifiers
Phthalic acid diamide (PDA) 5’-amino-modifiers were developed by Stefan Pitsch and ReseaChem Gmbh (Stefan Berger) and were introduced by Glen Research in Glen Report 24.1. PDA phosphoramidites have the huge advantage that they are chemically stable powders and can be shipped with no thermal protection even in high summer. In contrast, the equivalent TFA-protected monomers are viscous oils that are not simply handled, stored, or split into smaller aliquots by the customer. They are also susceptible to thermal degradation. The PDA protecting group was designed with methylamine and AMA in mind and is removed under the normal AMA deprotection conditions of 65°C for 10 minutes with no evidence of any of the side reactions that can occur when using the TFA-protected monomers. In addition, the PDA group is removed in AMA in only 30 minutes at room temperature.
In situations where trityl-on purification of the resulting amino-modified oligos is not necessary, the 5’-PDA-Amino-Modifiers are the clear winners for 5’-amino-modification. A comparison of all of our 5’-amino-modifiers was published in Glen Report 24.2.
Conclusion
When we introduced the UltraFAST chemistry for DNA synthesis and deprotection in conjunction with Beckman Instruments, we envisaged that it would become popular for the synthesis of simple unmodified DNA oligos. In combination with Ac-dC, this has indeed transpired, with this approach favored for high throughput oligo synthesis in most situations. However, we have also seen the use of AMA grow in popularity in more esoteric situations, which we have illustrated in this article with reference to RNA synthesis and amino-modification.

References
- M.P. Reddy, N.B. Hanna, and F. Farooqui, Tetrahedron Letters, 1994, 35, 4311-4314.
- M.P. Reddy, N.B. Hanna, and F. Farooqui, Nucleos Nucleot, 1997, 16, 1589-1598.
- M.P. Reddy, F. Farooqui, and N.B. Hanna, Tetrahedron Letters, 1995, 36, 8929-8932.
- S. Pitsch, P.A. Weiss, L. Jenny, A. Stutz, and X.L. Wu, Helv Chim Acta, 2001, 84, 3773-3795.
Product Information
Ac-dC-CE Phosphoramidite (10-1015)
C-TOM-CE Phosphoramidite (10-3014)
Ac-C-CE Phosphoramidite (10-3015)
- Glen Report 26.11: DNA conjugation to HaloTagged Protein Using Glen Bromohexyl Linker
- Glen Report 26.12: Preparation of Bromohexyl-Modified Oligonucleotides for HaloTag Conjugation
- Glen Report 26.13: New Product - 5-Me-dC Now Compatible with AMA and UltraMild Deprotection
- Glen Report 26.14: Technical Brief - AMA – Ubiquitous Reagent for Oligo Deprotection
- Glen Report 26.15: New Product - THPTA - A Water Soluble Click Ligand

