Glen Report 22.29: Technical Brief - Crosslinking with Click Chemistry
The click process is rapidly becoming the method of choice for key organic reactions useful in oligonucleotide modification.1 The advent of Copper (Cu1) catalyzed [3+2] azide-alkyne click chemistry (CuACC) has been the main reason for the surge in interest in this chemistry. CuACC reactions have simply improved the speed and performance of click chemistry. At the same time, the specificity of the reaction between an alkyne and an azide means that side reactions are very unlikely even in complex biological systems.
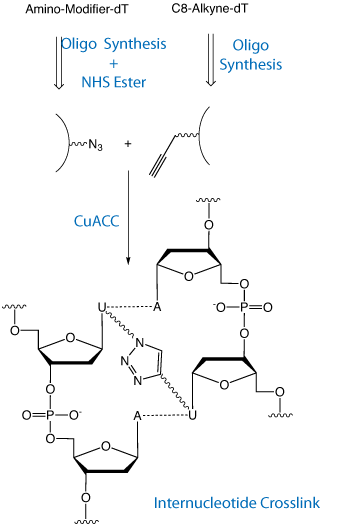
One of our most common questions from researchers is how to create a specific crosslink between the strands of an oligonucleotide duplex. Most procedures use light to create a crosslink using a free radical mechanism. Photolytic crosslinking is typically low yielding with many side reactions possible. Figure 1 shows click chemistry being used2 to form such an internucleotide crosslink. One DNA strand is simply modified using C8-Alkyne-dT (10-1540). The complementary strand is first modified using Amino-Modifier C6-dT (10-1039). A post-synthesis conjugation with Azidobutyrate NHS ester (50-1904) leads to the azido-modified strand. A subsequent CuACC click reaction leads to the intranucleotide crosslink in high yield.
In a recent article, this crosslinking technique was extended to hairpin and hammerhead ribozymes.3
References
- A.H. El-Sagheer, and T. Brown, Chem Soc Rev, 2010, 39, 1388-405.
- P. Kocalka, A.H. El-Sagheer, and T. Brown, Chembiochem, 2008, 9, 1280-1285.
- A.H. El-Sagheer, and T. Brown, Proc Natl Acad Sci U S A, 2010, 107, 15329-34.
Product Information
- Glen Report 22.21: 2’-F-Arabinonucleic Acid (2’-F-ANA)
- Glen Report 22.22: New Products – dX and Ferrocene-dT
- Glen Report 22.23:; New Product: 0.5M CSO for non-aqueous oxidation in DNA synthesis
- Glen Report 22.24: New Product - Universal Tosyl Phosphoramidite
- Glen Report 22.25: New Products - Glen Gel-Pak™ DNA/RNA Desalting and 1000Å Glen UnySupport™ Frits
- Glen Report 22.26: New Products – Azide Tags for Click Chemistry
- Glen Report 22.27: Deprotection – Volume 5 – On-Column Deprotection of Oligonucleotides in Organic Solvents
- Glen Report 22.28: Technical Brief - Symmetrically Branched Four-Arm DNA
- Glen Report 22.29: Technical Brief - Crosslinking with Click Chemistry
- Glen Report 22.210: Glen-Pak™ Product Update: New Protocols for Purification

