Glen Report 3-23: Rapid Trityl Colour Analysis Using a Fibre-Optic Probe
Richard T. Pon, Ph.D. Director, Regional DNA Synthesis Laboratory University of Calgary, Calgary, Alberta, Canada, T2N 4N1
The orange trityl cation released during the acidic deprotection step of oligonucleotide synthesis provides a rapid and quantitative signal of the efficiency of a solid-phase synthesis. Visual inspection of the colour intensity can readily detect gross failures (coupling1This digital colorimeter uses a flexible fibre-optic dipping probe which eliminates the need for sample transfer into cuvettes. This probe, along with a filter which measures absorbance off the peak maximum permits measurements to be made in the original collection tubes without the need for serial dilution. The PC-800 is much less expensive than a spectrophotometer and much faster to use.
Trityl colours are collected in polypropylene tubes (12ml) and diluted with 5% dichloroacetic acid/1 ,2- dichloroethane solution2 (5ml), dispensed from a bottle-top dispenser. The exact volume is not critical as long as each tube is diluted identically. After mixing, the colours are measured using the standard 2 cm path-length stainless steel probe supplied with the colorimeter. Measurement with either 470nm or 545nm filters for 0.2 or 1 micromole scale syntheses, respectively, will give absorbance readings and quantitation of every coupling step in the synthesis can be quickly accomplished. Stepwise coupling yields can then be easily calculated by comparing successive values. Alternatively, molar quantities can be estimated using respective extinction coefficients of 1.3 and 7.9 ml micromole-1 cm-1, for the absorbances at 545 and 470nm.
Although measuring the absorbance off the peak maximum of 505nm is convenient, the absorbance versus concentration relationship is not linear (see Figure). For precise work a calibration curve can be constructed, however for most applications the nonlinearity can be neglected as long as measurements are made in the most linear part of the curve (< 0.8 absorbance units).
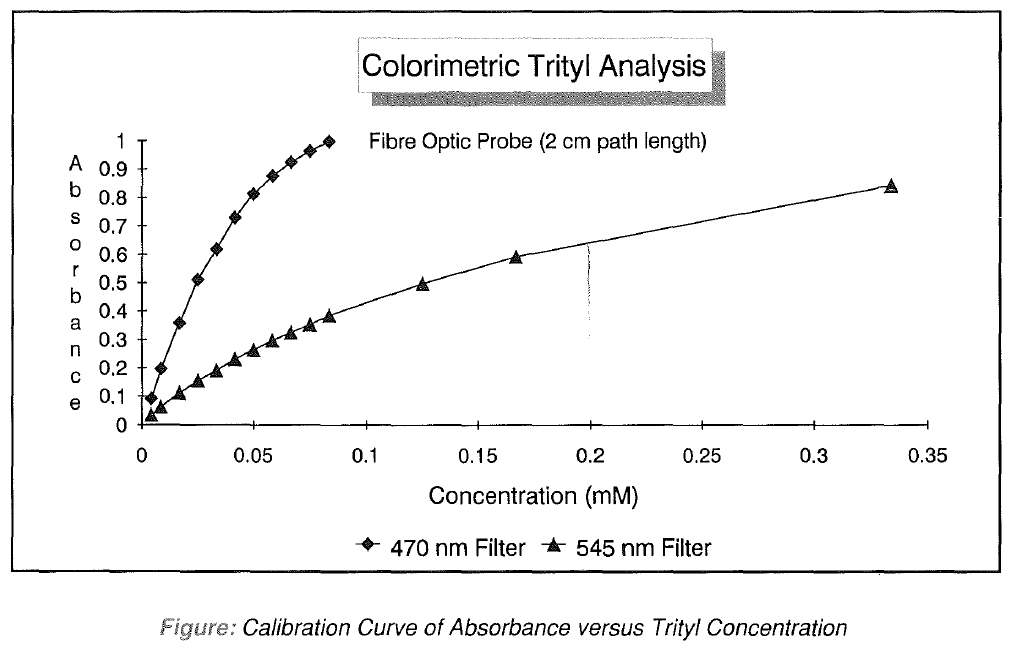
In our laboratory, this assay is routinely used on a daily basis to monitor the sequences produced. A simple computer program converts the absorbance data into step yields and also lists the average yields for each base. This regular monitoring allows us to quickly detect small decreases in coupling yields which may be indicative of pending instrument or reagent failure. This type of assay is also extremely useful for evaluating new procedures or synthesis programs because it allows much easier quantitation of every coupling step than analysis with a conventional spectrophotometer.
Notes:
1 . The PC 800 Probe Colorimeter and accessories are available from Brinkmann Instruments, Inc., Cantiague Road, Westbury, NY 11590;(516)334-7500,
2. In our laboratory, 1,2-dichloroethane is preferred over dichloromethane for the detritylating reagent because it is less volatile and less toxic. However, the above procedure will also work with solutions of trichloroacetic acid in dichloromethane.

