Glen Report 21.24: Metallobase Nucleic Acid Modification
Exciting new technologies have emerged from the capability to manipulate oligonucleotides using transition metal coordinating organic ligands covalently linked to DNA. Chelate-ligand modified oligonucleotides have been used for in vivo siRNA imaging,1 or sequence selective DNA cleavage.2 Researchers have prepared metallo-DNA supramolecular assemblies that raise the fascinating possibility of metal-based molecular nano-devices such as molecular scale magnets and wires.3,4 Metallobases can be used to precisely control modified DNA through the addition (or removal) of a small amount of selected metal ion, which may lead to astonishing advancements in oligonucleotide research. Several groups have already reported exceptionally stable unnatural metallobase pairs using ligand modified oligonucleotides.5-8
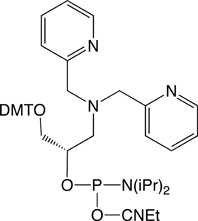
Each unique chelation complex exhibits a distinct character regarding affinity and specificity of metal-ion coordination and interaction with the DNA backbone or nucleobases. 2,2’-Dipicolylamine is a versatile metal-coordinating ligand capable of forming complexes with common metal ions including Zn2+, Ni2+, Cu2+, or Ag+.9 A tremendous advantage of dipicolylamine is complete compatibility with standard DNA synthesis, cleavage and purification protocols. Other chelating ligands may require nonstandard conditions or additional protection and deprotection steps.
Dipicolylamine-metal complexes have been used for the biochemical detection of phosphate10 and zinc.11 Kady and Groves used dipicolylamine-Fe2+ modified oligonucleotides for the sequence-specific cleavage of a DNA target.12 Other research groups have proposed using the dipicolylamine ligand for chelation of 99mTc or 188Re for biomolecular imaging and therapy.13,14 Dipicolylamine modified oligonucleotides may also find exciting and innovative uses for surface or nano-particle functionalization, real-time PCR, DNA microarrays, or antisense therapeutics.
We are pleased to offer 2,2’-dipicolylamine phosphoramidite. This product was manufactured and developed by Syntrix Biosystems Inc. Patents Pending. For Research Use Only.
References:
- Bartlett, D. W.; Su, H.; Hildebrandt, I. J.; Weber, W. A.; Davis, M. E., Impact of tumor-specific targeting on the biodistribution and efficacy of siRNA nanoparticles measured by multimodality in vivo imaging. Proc Natl Acad Sci U S A 2007, 104, (39), 15549-54.
- Dreyer, G. B.; Dervan, P. B., Sequence-specific cleavage of single-stranded DNA: oligodeoxynucleotide-EDTA X Fe(II). Proc Natl Acad Sci U S A 1985, 82, (4), 968-72.
- Tanaka, K.; Tengeiji, A.; Kato, T.; Toyama, N.; Shionoya, M., A discrete self-assembled metal array in artificial DNA. Science 2003, 299, (5610), 1212-3.
- Shionoya, M.; Tanaka, K., Artificial metallo-DNA: a bio-inspired approach to metal array programming. Curr Opin Chem Biol 2004, 8, (6), 592-7.
- Weizman, H.; Tor, Y., 2,2’-Bipyridine ligandoside: a novel building block for modifying DNA with intra-duplex metal complexes. J Am Chem Soc 2001, 123, (14), 3375-6.
- Atwell, S.; Meggers, E.; Spraggon, G.; Schultz, P. G., Structure of a copper-mediated base pair in DNA. J Am Chem Soc 2001, 123, (49), 12364-7.
- Clever, G. H.; Kaul, C.; Carell, T., DNA--metal base pairs. Angew Chem Int Ed Engl 2007, 46, (33), 6226-36.
- Müller, J., Metal-ion-mediated base pairs in nucleic acids. Eur. J. Inorg. Chem. 2008, 3749-3763.
- Martell, A. E., Critical Stability Constants. Plenum Press: New York, 1974.
- Sakamoto, T.; Ojida, A.; Hamachi, I., Molecular recognition, fluorescence sensing, and biological assay of phosphate anion derivatives using artificial Zn(II)-Dpa complexes. Chem Commun (Camb) 2009, (2), 141-52.
- Lim, N. C.; Bruckner, C., DPA-substituted coumarins as chemosensors for zinc(II): modulation of the chemosensory characteristics by variation of the position of the chelate on the coumarin. Chem Commun (Camb) 2004, (9), 1094-5.
- Kady, I. O.; Groves, J. T., Synthesis of Iron-Binding Oligonucleotides and Their Reactions with Single-Stranded DNA. Biorg. Med. Chem. Lett. 1993, 3, 1367-1370.
- Liu, G.; Dou, S.; He, J.; Vanderheyden, J. L.; Rusckowski, M.; Hnatowich, D. J., Preparation and properties of 99mTc(CO)3+-labeled N,N-bis(2-pyridylmethyl)-4-aminobutyric acid. Bioconjug Chem 2004, 15, (6), 1441-6.
- Storr, T.; Sugai, Y.; Barta, C. A.; Mikata, Y.; Adam, M. J.; Yano, S.; Orvig, C., Carbohydrate-appended 2,2’-dipicolylamine metal complexes as potential imaging agents. Inorg Chem 2005, 44, (8), 2698-705.
Product Information
2,2'-Dipicolylamine Phosphoramidite has been discontinued.
- Glen Report 21.21: 5-Hydroxymethyl-dC: A New Actor in the Field of Epigenetics
- Glen Report 21.22: Purification of CleanAmp™ DNA Oligonucleotides (DMT-ON)
- Glen Report 21.23: A 3'-Cap for Improved Target Affinity and Specificity
- Glen Report 21.24: Metallobase Nucleic Acid Modification
- Glen Report 21.25: tC and tCo: New Tricyclic Fluorescent Cytidine Analogues with a very Bright Future
- Glen Report 21.26: Non-Aqueous Oxidation for PACE Chemistry
- Glen Report 21.27: New Product - Azobenzene Phosphoramidite for the Introduction of Photo-regulated Functions in DNA
- Glen Report 21.28: Deprotection - Volume 3 - Dye-Containing Oligonucleotides
- Glen Report 21.29: New Products - Glen UnySupport™ Frits
- Glen Report 21.210: New products: Glen-Pak™ DNA Cartridge 3g and DNA 30mg 96-Well Plates
- Glen Report 21.211: Technical Brief – Synthesis of Long Oligonucleotides

