Glen Report 14.12: A New Simplified 3'-Amino-Modifier CPG
Introduction
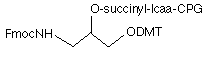
The use of oligonucleotides modified with aliphatic amino groups continues to grow as immobilization to surfaces becomes even more important than labelling with tags. The selection of commercially available 3'-amino-modifiers has been dominated by two products1,2 containing branched linkers in which the amino group is protected with the fluorenylmethoxycarbonyl (Fmoc) group. These are supplied by Glen Research and are the products 3'-Amino-Modifier C3 CPG (1) and 3'-Amino-Modifier C7 CPG (2), as shown in Figure 1. Since the Fmoc group is base-labile, it offers some advantages but also disadvantages.
.gif)
Advantages
- The Fmoc group is quite stable and allows synthesis to proceed without significant loss.
- The Fmoc group can be removed specifically from the support to allow solid-phase conjugation of the desired tag. This can be done before or after the oligonucleotide synthesis.
- These supports have been used effectively and successfully for more than 10 years.
Disadvantages
- If the support is not handled properly, some loss of Fmoc group can occur. The free amino group is then capped with acetic anhydride and the resulting acetyl group is not removed during deprotection, leading to lower conjugation yields.
- Because of their branched structures, the linkers each contain a chiral center which generates a pair of diastereomers after oligo synthesis and these may be separated during HPLC analysis and purification.
DMT-Amino C6-CPG
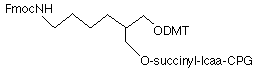
An amino support without an Fmoc group has been described by Lyttle and coworkers3 and is now commercially available (Biosearch Technologies, Inc.). Under normal circumstances, an aliphatic amide cannot be simply hydrolyzed under conditions appropriate for oligonucleotide deprotection. The novel support (3) contains an amide group which is hydrolyzed to give the aliphatic amine because of the participation in the hydrolysis of the neighboring carboxylic acid group. This approach does require extended cleavage conditions (ammonium hydroxide at 55°C for 17 hours) but the deprotection of the bases is obviously achieved simultaneously.
3'-PT-Amino-Modifier C6 CPG
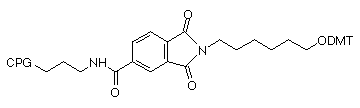
Another interesting approach was described in 19924 and has remained commercially dormant since then. In this approach, the nitrogen destined to become the 3'-amino group is included in a phthalimide (PT) group which is attached to the support through an amide group attached to the aromatic ring. The structure of the support (4) is shown in Figure 1. This simple linkage is very stable to all conditions of oligonucleotide synthesis and contains no chiral center. Again, using an extended ammonium hydroxide treatment (55°C for 17 hours), the cleavage of the amine from the phthalimide is accomplished along with the deprotection of the oligonucleotide.
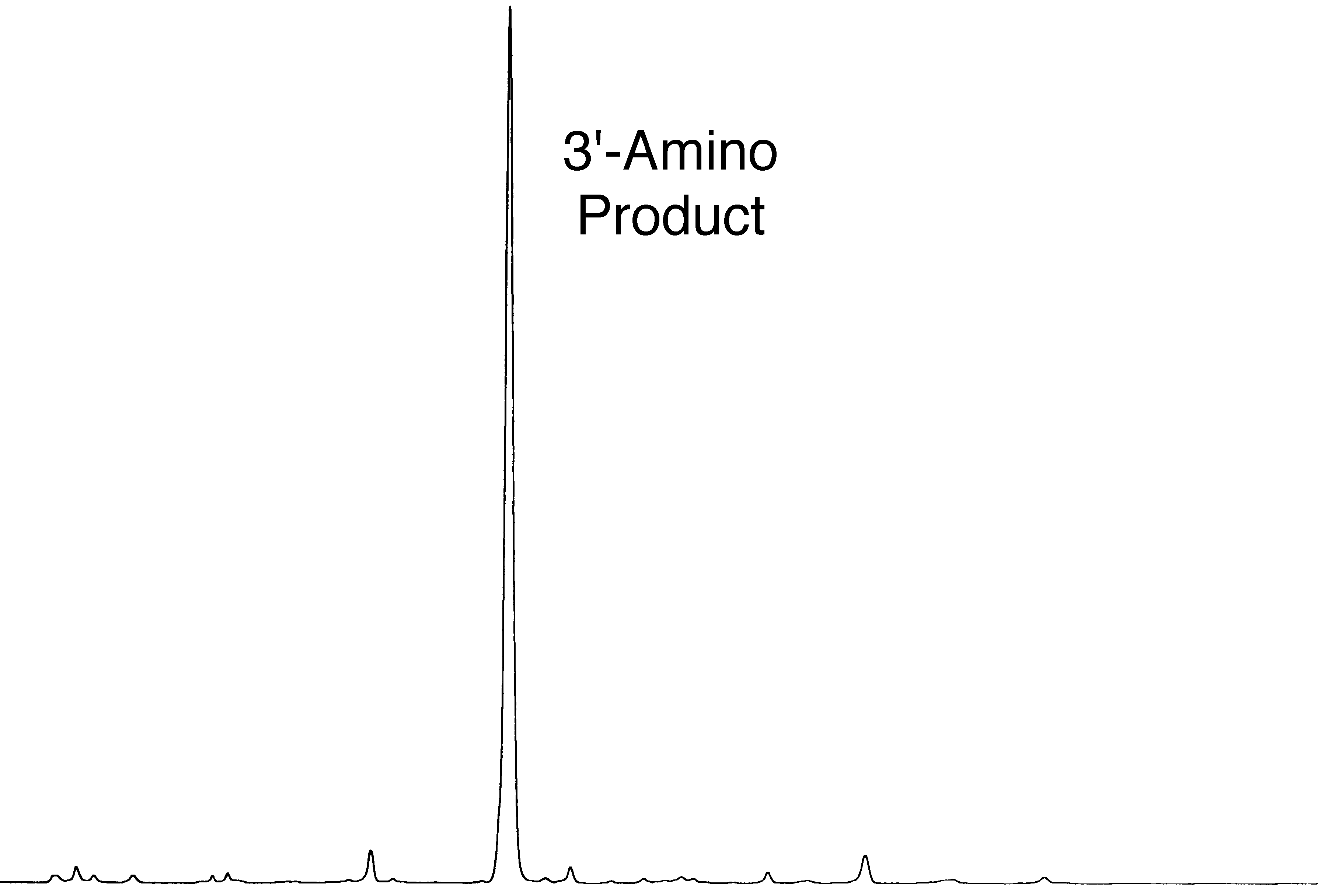
A comparison of the yields of crude oligonucleotides produced with 3'-Amino-Modifier C7 CPG (2) and 3'-PT-Amino-Modifier C6 CPG is shown in Table 1 and the HPLC purity of the oligonucleotides is shown in Figure 2 and Figure 3. The results indicate that the yield of product from the 3'-PT-Amino-Modifier C6 CPG is about 20% lower if deprotected with ammonium hydroxide. However, the purity of amino-modified product is significantly higher due to the absence of the acetyl capped product.

References
- P.S. Nelson, R. Sherman-Gold, and R. Leon, Nucleic Acids Res., 1989, 17, 7179-86.
- P.S. Nelson, M. Kent, and S. Muthini, Nucleic Acids Res., 1992, 20, 6253-6259.
- M.H. Lyttle, H. Adams, D. Hudson, and R.M. Cook, Bioconjugate Chemistry, 1997, 8, 193-198.
- C.R. Petrie, M.W. Reed, A.D. Adams, and R.B. Meyer, Jr., Bioconjugate Chemistry, 1992, 3, 85-7.
Product Information
3'-Amino-Modifier C3 CPG has been discontinued.
3'-Amino-Modifier C7 CPG 500 has been discontinued.
3'-Amino-Modifier C7 CPG 1000 (20-2958)
3'-PT-Amino-Modifier C6 CPG (20-2956)
- Glen Report 14.11: Novel Universal Support Features Rapid Amide-Assisted Dephosphorylation
- Glen Report 14.12: A New Simplified 3'-Amino-Modifier CPG
- Glen Report 14.13: Preparation of Oligonucleotides Containing Abasic Sites
- Glen Report 14.14: PC Biotin and Related Photocleavable Modifiers
- Glen Report 14.15: New OPeC™ Reagents for Synthesis by Native Ligation of Oligonucleotide-Peptide Conjugates
- Glen Report 14.16: Product Update - How Are Methyl Triester Linkages Prepared?

