DNA-peptide conjugates have, in recent years, been identified as valuable tools in molecular biology. In particular, they have become increasingly important due to the identification of peptides as viable carriers for enhancing the cell delivery of oligonucleotides.1
Unfortunately, the hitherto cumbersome and inefficient methods of preparing DNA-peptide conjugates have hampered the systematic study of the sequence and structural requirements for good cell penetration. Total step-wise solid-phase synthesis on a single support has posed serious difficulties because of the incompatibilities in peptide and oligonucleotide protection and assembly chemistries. Increasingly, then, it has become clear that a post-assembly conjugation strategy offers the widest possibilities
Awareness of this has led to the introduction of a new range of OPeC™ reagents for the synthesis of Oligonucleotide-Peptide Conjugates. These reagents have been developed by Link Technologies Ltd in Scotland () and now Glen Research is pleased to be able to supply these products to our customers
In OPeC™ conjugation strategies, peptide and oligonucleotide units are easily assembled separately on their own supports using conventional synthesizers and methodology. Each biomolecule is designed to carry a reactive functionality that is released upon full deprotection and cleavage from the support. The components can then be conjugated in aqueous/organic solution by selective reaction of these functionalities.
Prior to the emergence of OPeC™ technology, only a limited number of post-assembly conjugations had been reported. The range of methodologies used include the formation of a disulfide bond2 ,3 ,4 , reaction of a cysteine peptide with a maleimido oligonucleotide5 ,6 ,7 , and a bromoacetyl peptide with a thiol-functionalized oligonucleotide.8 There are a number of important disadvantages of these methods however. For example, a disulfide bond is unstable to reducing agents that may be present under many assay conditions or within cells. The maleimide-thiol route requires a functionalization step on the oligonucleotide portion after release from the solid support. In addition, and perhaps most seriously of all, all three routes may sometimes lead to inefficient conjugation due to secondary structure or poor stability of certain peptide components in aqueous solution.
The "native ligation" strategy9 of OPeC™ reagents has many attractions. First, the reagents used for the functionalization of the peptide and oligonucleotide components are stable, easy to handle solids. Second, the reagents are used in standard protocols of solid-phase synthesis and couple to their respective peptide or DNA/RNA partners in high yield. Third, assuming the peptide and oligonucleotide fragments are themselves prepared efficiently, the modified components may be used in the unpurified form for conjugation without the need for further manipulation following deprotection and release from their respective solid supports.
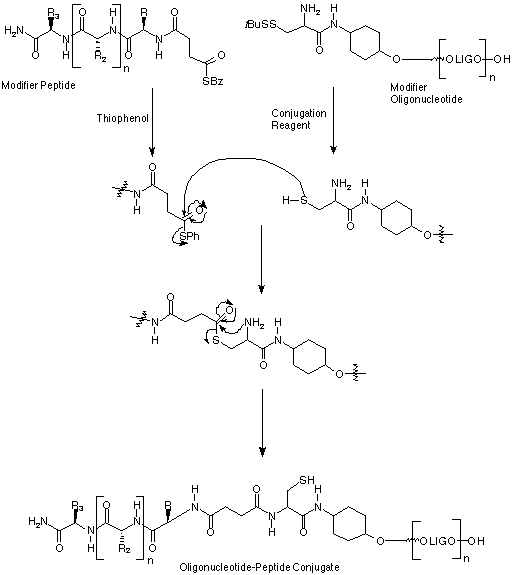
The OPeC™ method is based on the "native ligation" of an N-terminal thioester-functionalized peptide to a 5'-cysteinyl oligonucleotide. Pentafluorophenyl S-benzylthiosuccinate, Peptide Modifying Reagent (PMR), (1) in Figure 1, is used in the final coupling step in standard Fmoc-based solid-phase peptide assembly. Deprotection with trifluoroacetic acid generates, in solution, peptides substituted with an N-terminal S-benzylthiosuccinyl group (Modified Peptide in Scheme 1).
O-trans-4-(N-a-Fmoc-S-tert-butylsulfenyl-l-cysteinyl)aminocyclohexyl O-2-cyanoethyl-N,N-diisopropylphosphoramidite, (2) Oligonucleotide Modifying Reagent (OMR), (2) in Figure 1, is used in the final coupling step in standard phosphoramidite solid-phase oligonucleotide assembly. Deprotection with aqueous ammonia solution generates in solution 5'-S-tert-butylsulfenyl-L-cysteinyl functionalized oligonucleotides (Modified Oligonucleotide in Scheme 1).
The thiobenzyl terminus of the Modified Peptide is converted to the thiophenyl analogue by the use of thiophenol, whilst the Modified Oligonucleotide is reduced using the tris(carboxyethyl)phosphine (Conjugation Reagent in Scheme 1). Coupling of these two intermediates, followed by the "native ligation" step, leads to formation of the Oligonucleotide-Peptide Conjugate.

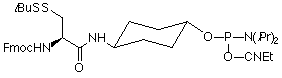
Oligonucleotide-Peptide Conjugation Kit (OPeC) has been discontinued