Glen Report 21.22: Purification of CleanAmp™ DNA Oligonucleotides (DMT-ON)
In the previous Glen Report 21.1, we were happy to launch CleanAmp™ monomers for the production of Turbo and Precision Primers under license from TriLink BioTechnologies, Inc. Customer feedback has indicated that the purification scheme which we detailed, while successful, is quite tedious, with large volumes of solutions being pushed slowly through Sep-Pak® Cartridges. In the past few months, we have been working to optimize this procedure using Sep-Pak cartridges, as well as our Poly-Pak™ cartridges, representing older OPC-type cartridges, and our Glen-Pak™ cartridges, representing the most up-to-date pH-stable purification cartridges.
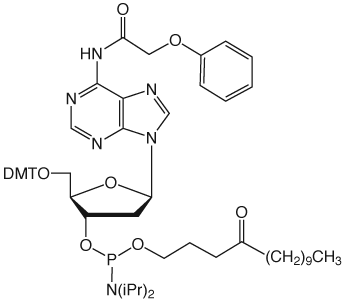
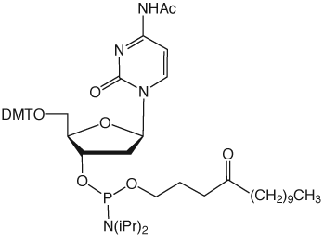
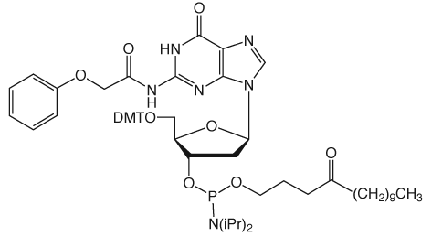
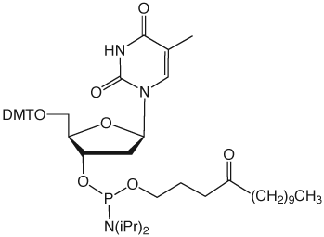
Unfortunately, Poly-Pak cartridges gave primers of unacceptable purity but Sep-Pak and Glen-Pak cartridges gave both Turbo and Precision Primers of good quality following a much simplified procedure. The procedure is described below for our Glen-Pak cartridges.
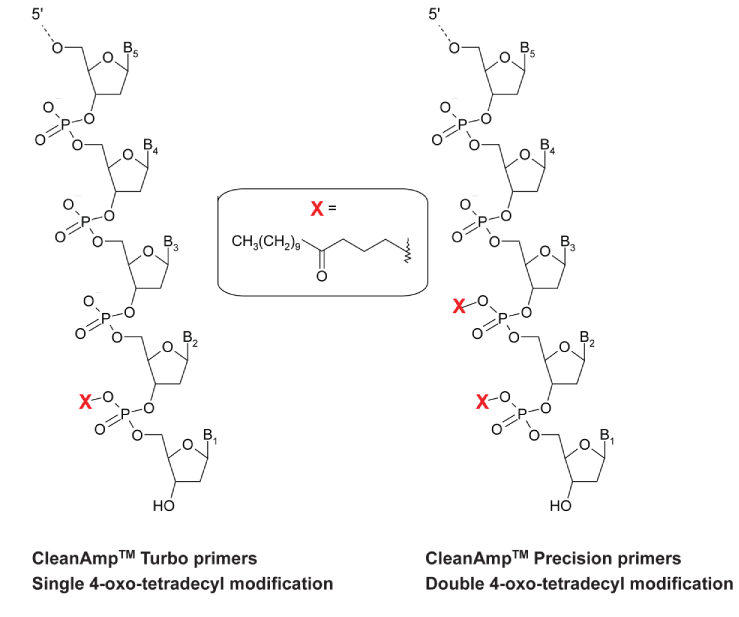
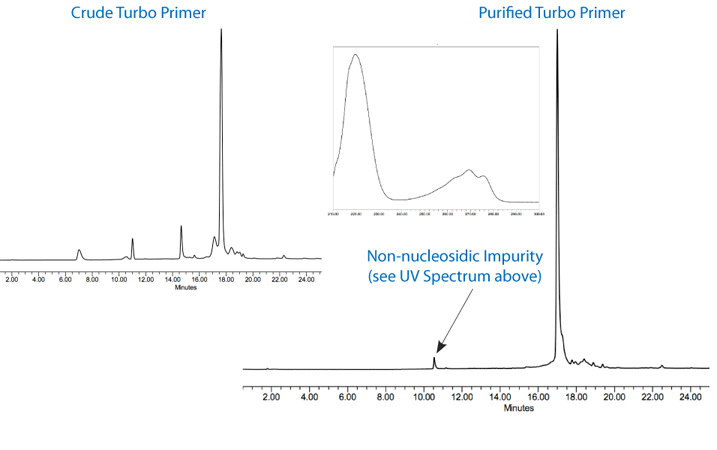
Turbo and Precision Primers were synthesized normally using CleanAmp™ Phosphoramidites (Figure 1) to generate the sequences shown in Figure 2. Crude and purified Turbo and Precision Primers are shown in Figures 3 and 4, respectively.

Procedure
Sample Preparation
| Materials | Amount Used |
|---|---|
| Glen-Pak™ DNA Purification Cartridge (60-5100-XX, 60-5200-XX) | 1 |
| 0.05 M Potassium Carbonate in Methanol (60-4600-30) | 1 mL |
| Vacuum Manifold or handheld 5 mL syringe | 1 |
| HPLC Grade Acetonitrile (ACN) | 2 mL |
| 2.0 M Triethylammonium Acetate (TEAA) (60-4110-57) | 1 mL |
| 1.0 M TEAA, prepared from 2.0 M TEAA | 1 mL |
| 2% Trifluoroacetic acid (TFA) in Water (60-4040-57) | 4 mL |
| Deionized Water | 1 mL |
| 0.1 M TEAA in water | 6 mL |
| 0.1 M TEAA containing 35% ACN‡ | 3 mL |
| 0.1 M TEAA containing 25% ACN‡,+ | 3 mL |
| 0.1 M TEAA containing 15% ACN+ | 3 mL |
| DMSO, anhydrous | 1 mL |
| For Precision‡ and Turbo+ Primers. Note: Prepare these solutions at a final concentration of 0.1 M TEAA using 2.0 M TEAA. Do not dilute 0.1 M TEAA with Acetonitrile. | |
- Following DNA synthesis using UltraMild monomers on either an UltraMild CPG or US III Universal Support, deprotect the DMT-ON oligonucleotide in 1 mL of 0.05 M potassium carbonate solution for 4 hours at room temperature. Just prior to beginning the purification of the CleanAmp Turbo or Precision primers on the Glen-Pak cartridge, quench the 0.05 M potassium carbonate with 1 mL of 1.0 M TEAA. Note: Once the purification is begun, avoid interruptions during the protocol; this is especially true when removing the DMT. If the purification cannot be completed the same day, store the oligo in the freezer in the original 0.05 M potassium carbonate solution until ready for purification.
Cartridge Preparation
- Rinse the cartridge with 2 mL of acetonitrile followed by 1 mL of 2.0 M TEAA. The acetonitrile wets the Glen-Pak resin, while the TEAA acts as an ion-pairing reagent to enhance the binding of the DMT-ON oligonucleotide to the resin.
Purification Procedure
- Load the CleanAmp primer in the quenched potassium carbonate/TEAA onto the prepped Glen-Pak cartridge dropwise. Collect the eluent and save in case of a loading failure or error. During the loading process, the DMT-ON Triesters bind to the resin while the failure sequences do not.
- Wash the cartridge with 2 mL of 0.1 M TEAA. This removes the salts and methanol from the cartridge.
- Turbo Primers
Rinse the cartridge with 3 mL of 25% ACN in 0.1 M TEAA. This rinses away DMT-Off failures and hydrolyzed Diester DMT-ON sequences. - Precision Primers
Rinse the cartridge with 3mL of 35% ACN in 0.1 M TEAA. This rinses away DMT-Off failures and mixed Diester/Triester DMT-ON sequences.
- Turbo Primers
- Rinse cartridge with 2 mL 2% TFA dropwise. Repeat. This removes the DMT. The faint orange color of the DMT cation is often visible at this step.
- Rinse the cartridge with 2 mL of 0.1 M TEAA. This removes residual TFA and neutralizes the resin.
- Turbo Primers
Rinse the cartridge with 3 mL of 15% ACN in 0.1 M TEAA. This rinses away Diesters that are produced during detritylation. - Precision Primers
Rinse the cartridge with 3 mL of 25% ACN in 0.1 M TEAA. This rinses away Diesters and mixed Diester/Triesters that are produced during detritylation.
- Turbo Primers
- Rinse the cartridge with 2 mL of 0.1 M TEAA. This removes residual ACN.
- Rinse the cartridge with 1 mL of water. This removes excess TEAA.
- Using one syringe volume of air, blow out any residual water.
- With a clean, dry syringe, elute the oligonucleotide in three fractions of DMSO, collecting each fraction separately. After collecting a fraction, blow the residual DMSO into the collection tube after each aliquot using a syringe full of air.
- 1st fraction 300 µL of DMSO; removes water and remaining failure sequences.
- 2nd fraction 400 µL of DMSO; elutes the bulk of the oligo
- 3rd fraction 300 µL of DMSO; elutes the remaining oligo
- Determine ODs of each fraction. Generally, fraction 2 is the most pure and contains the majority of the Turbo or Precision CleanAmp primer. If the concentration is low, add a portion of fraction 3 to fraction 2 as necessary to increase the amount of CleanAmp primer. Fraction 1 generally contains excess water that will reduce the long-term stability of the CleanAmp primers.
Product Information
CleanAmp™ products have been discountinued.
- Glen Report 21.21: 5-Hydroxymethyl-dC: A New Actor in the Field of Epigenetics
- Glen Report 21.22: Purification of CleanAmp™ DNA Oligonucleotides (DMT-ON)
- Glen Report 21.23: A 3'-Cap for Improved Target Affinity and Specificity
- Glen Report 21.24: Metallobase Nucleic Acid Modification
- Glen Report 21.25: tC and tCo: New Tricyclic Fluorescent Cytidine Analogues with a very Bright Future
- Glen Report 21.26: Non-Aqueous Oxidation for PACE Chemistry
- Glen Report 21.27: New Product - Azobenzene Phosphoramidite for the Introduction of Photo-regulated Functions in DNA
- Glen Report 21.28: Deprotection - Volume 3 - Dye-Containing Oligonucleotides
- Glen Report 21.29: New Products - Glen UnySupport™ Frits
- Glen Report 21.210: New products: Glen-Pak™ DNA Cartridge 3g and DNA 30mg 96-Well Plates
- Glen Report 21.211: Technical Brief – Synthesis of Long Oligonucleotides

