Glen Report 27.17: Technical Brief - DBCO-dT - An Unusual Case of Iodine Sensitivity
The Dibenzocyclooctyne (DBCO) family of products allows "click" conjugation to an azide simply and cleanly without requiring any copper salts or chelators, making it very convenient - and especially useful for in vivo conjugation reactions. After our successful launch of the 5'-DBCO-TEG Phosphoramidite and NHS ester, we later added to our portfolio DBCO-dT (1) which allows internal modification of an oligo with DBCO using a 5-substituted thymidine analog.
Recently, however, we received information from a customer in Europe, Microsynth AG, indicating that full-length production of a poly-dT 63-mer with 3 incorporations of the DBCO-dT could not be obtained in good yield. According to their mass spec data, it appeared that the DBCO had been cleaved off the linker. When the same batch of DBCO-dT was retested here, the coupling efficiency was essentially quantitative and the 12-mer test oligo with 3 incorporations of the DBCO-dT looked excellent, as shown in Figure 2a.
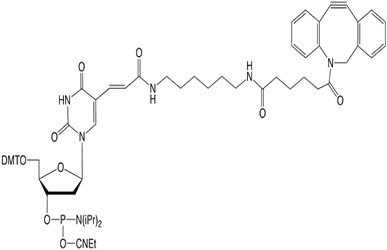
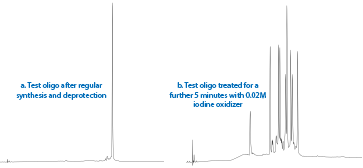
Since the performance of this batch of DBCO-dT looked good, as a courtesy, we offered to synthesize the customer's longer sequence. However, when analyzed by RP HPLC, the resulting oligonucleotide looked very impure and mass spec analysis confirmed that the DBCO had been substantially cleaved off the linker.
Conceptually, this was unexpected since amide linkages are resistant to hydrolysis, which implied that DBCO-dT is sensitive to one or more of the synthesis reagents and that the repeated exposure during the synthesis of long oligos led to cleavage of the DBCO. To test this hypothesis, CPG from the 12-mer synthesis (Figure 2a) was subjected to treatment with standard DNA synthesis oxidizer, 0.02 M Iodine, for 5 minutes at room temperature. This exposure is equivalent to roughly 20 synthesis cycles. As shown in Figure 2b, the resulting degradation was quite dramatic.
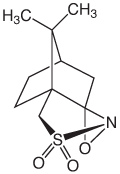
With other nucleoside analogs with sensitivity to iodine, we have achieved good results using an alternative oxidizer, (1S)-(+)-(10-Camphorsulfonyl)-oxaziridine (CSO) (2).
So, when the 63-mer was re-synthesized using 0.5 M CSO in acetonitrile (40-4632-xx) and a 3 minute oxidation time, the hoped-for improvement was dramatic. Figure 3 shows the deconvolved electrospray MS data for the same sequence synthesized using standard 0.02 M Iodine versus 0.5 M CSO with the target mass being 20,511 Da. It is clear that the DBCO moiety is being cleaved off when exposed to iodine-based oxidizers. What appears to have occurred during oxidation with iodine is the formation of an N-iodo amide, making the amide linkage unstable. During deprotection, the DBCO is cleaved off, leaving a hexamido linker present. (The splitting of the -DBCO peaks is 14 Da, indicating the formation of both the amide and N-methylamide linkers which results from the oligo being deprotected in AMA). The lower molecular weight peaks associated with the CSO-oxidized oligo are deletion mutants (-1, -2 and -3 dTs), which suggests the oxidation time of 3 minutes should have been increased slightly for an oligo of this length.
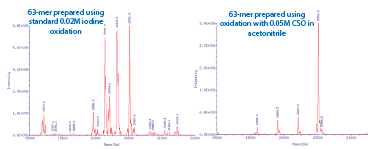
As a result of these data, we now recommend that synthesis of oligos containing DBCO-dT be completed using 0.5 M CSO oxidizer. Acceptable results can be achieved with iodine oxidation if DBCO-dT is subjected to no more than 8-10 cycles. We thank Microsynth AG for bringing this issue to our attention.
Ordering Information
DBCO-dT-CE Phosphoramidite (10-1539)
0.5M CSO in Anhydrous Acetonitrile (40-4632)
- Glen Report 27.11: Reversible Photo-Switching of DNA Function with Azobenzene-Tethered DNA
- Glen Report 27.12: New Products - Dithiol Serinol Phosphoramidite and 3’- Dithiol Serinol CPG
- Glen Report 27.13: Technical Brief - APA: An Alternative to AMA Deprotection
- Glen Report 27.14: Photocleavable Biotin Linker for Use in SOMAscan™
- Glen Report 27.15: PC Modifiers
- Glen Report 27.16: Technical Brief - Capping and Trityl-Protected Amino-Modifiers
- Glen Report 27.17: Technical Brief - DBCO-dT - An Unusual Case of Iodine Sensitivity

