Glen Report 27.11: Reversible Photo-Switching of DNA Function with Azobenzene-Tethered DNA
Yukiko Kamiya1,2 and Hiroyuki Asanuma2
- EcoTopia Science Institute, Nagoya University
- Department of Molecular Design and Engineering, Graduate School of Engineering, Nagoya University
Deoxyribonucleic acids are recognized not only as biomolecules encoding genetic information but also as structural materials in nanotechnology due to their supramolecular properties. The ability to control DNA properties artificially using external stimuli offers great potential for a wide variety of applications. Among external stimuli, light has several advantages: (1) unlike molecular stimuli, light does not contaminate the microenvironment of the reaction system; (2) spatiotemporal control of the reaction is possible; and (3) irradiation wavelengths are tunable by suitable molecular design. In this report, we introduce the photo-responsive DNA that we developed over the past decade.
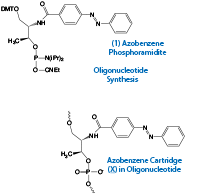
Introduction of azobenzene into DNA-like cartridges
Azobenzene derivatives are the most popular photo-responsive molecules for versatile applications because of their ready availability and chemical stability. A planar trans form can be obtained upon photo-irradiation at wavelengths > 400 nm and a nonplanar cis form is obtained by photo-irradiation at 300 nm - 400nm. Therefore, azobenzene can reversibly photo-isomerize between trans and cis forms upon irradiation with the appropriate wavelength of light. On the basis of this property, azobenzene can be used as a photo-switch in a DNA-based nanomachine or a DNA-mediated bioprocess. By synthesizing a cartridge-like unit of azobenzene (Figure 1a),1 we have attained 1) photo-regulation of the formation and dissociation of a DNA duplex (Figure 1b), and 2) photo-regulation of transcription and translation with photo-responsive T7 promoter (Figure 1c).2,3
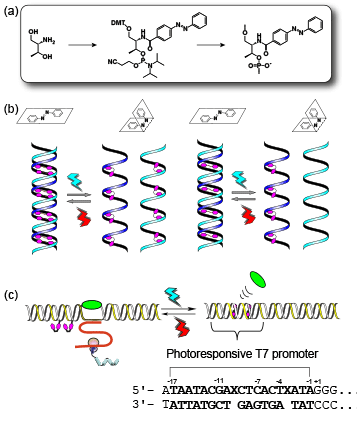
(a) Azobenzene amidite synthesized from D-threoninol for introducing an azobenzene unit into DNA. (b) Reversible photo-regulation of formation and dissociation of the duplex with photo-responsive DNA containing multiple azobenzene units. (c) Reversible photo-switching of transcription by photo-responsive T7 promoter containing two azobenzene units.
In our methods, we synthesized a phosphoramidite monomer (1) bearing an azobenzene group covalently connected through an amide bond to D-threoninol as the scaffold (above and Figure 1a on Page 2). Use of this monomer allows the simple introduction of an azobenzene cartridge into DNA. To provide the functionality of azobenzene in DNA, the azobenzene cartridge (X) is introduced between base pairs of the DNA. For example, if we want to provide 5’-GCGAGTCG-3’ with photo-responsiveness, the X residue is introduced to obtain, e.g., 5’-GCGAXGTCG-3’. The modified DNA strand can still form a duplex with its complementary strand, 3’-CGCTCAGC-5’ and all the base pairs are maintained in the duplex. It should be noted that replacing a natural nucleotide with X is not recommended as it causes destabilization of the duplex.4 Our NMR analyses revealed that trans-azobenzene, which is planar, stabilizes the double-stranded DNA as it intercalates in the duplex and that the non-planar cis-azobenzene destabilizes the duplex due to steric hindrance.4 In order to attain efficient photo-regulation, introduction of multiple azobenzene residues is effective, such as 5’-GCXGAXGTXCG-3’/3’-CGCTCAGC-5’ (right panel of Figure1b). If both strands can be modified, sequence design like 5’-GCXGAXGTXCG-3’/3’-CXGCXTCXAGXC-5’ is particularly effective (left panel of Figure1b). For the introduction of multiple azobenzenes, at least two nucleotides should be inserted between the X residues. Using these design strategies, repetitive and efficient regulation of hybridization and dissociation of the DNA duplex can be promoted by alternating between UV and visible light. In addition to control of DNA duplexes, photo-control of the hybridization of DNA/RNA and RNA/RNA duplexes and triplex DNA can also be achieved by introducing azobenzene as a photo-responsive switch. 5,6
Light driven DNA nano-machine with a photo-responsive molecular engine
Recently, DNA has been recognized as a useful material in the field of nanotechnology. From the viewpoint of constructing DNA-based nanomachines, modulating DNA hybridization in response to light provides an interesting application. By using azobenzene modified DNA, we demonstrated a photon-driven DNA nanomachine. We designed a photo-responsive DNAzyme which changes its structure between open and closed forms upon light irradiation in a tweezer-like motion (Figure 2).7 To make open and closed forms of the DNAzyme, extended azobenzene-modified DNA regions were attached at both the 3’ and 5’ ends of the DNAzyme. In this design, we introduced multiple azobenzenes to increase photo-responsiveness. The DNAzyme with trans-azobenzenes was inactive due to being in the closed state caused by hybridization of the extended regions. Upon cis-isomerization of the azobenzenes, the extended regions are dissociated and the DNAzyme returned to the active form, inducing RNA cleavage. Alternate irradiation with UV and visible light clearly caused on-off photo-switching of RNA cleavage.
Photo-regulation of transcription by a photo-responsive promoter
Artificial control of biofunctions by external stimuli is one of the current hot topics in chemical biology or synthetic biology. Using azobenzene, a photo-triggered gene expression system can be constructed. Isomerization of azobenzenes in the modified T7 promoter allows reversible photo-regulation of the transcription reaction by T7-RNA polymerase (Figure 1c).8 This regulation is based not on photo-regulated hybridization but on a local structural change of the DNA duplex induced by photo-isomerization of azobenzenes between the trans and cis forms, allowing control of the DNA-protein interaction.
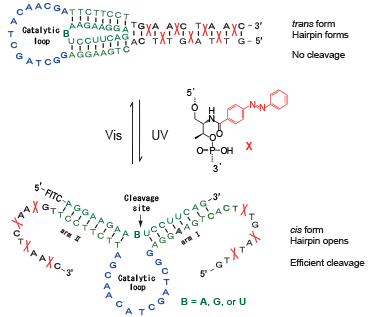
Photo-responsive DNAzyme that can perform tweezer-like motion by light-irradiation and achieve on-off cleavage of RNA.
We have developed an effective photo-regulation design. In this design, the template strand remains intact and two azobenzenes are introduced into the non-template strand of the T7 promotor region. One azobenzene is inserted between -3 and -4 and another is inserted between -9 and -10 in the non-template strand of the T7 promoter region (Figure 1c). The DNA duplex does not dissociate when the azobenzenes are in the cis form even though the local structure of the duplex around azobenzene is altered. If the azobenzenes are in the trans form, transcription is terminated by inhibition of binding of the polymerase with the promoter region. Upon cis-isomerization of azobenzene, transcription is switched on because the unwound promoter region facilitates binding of the polymerase. This change of the binding mode of the polymerase to the photo-responsive promoter allows reversible photo-regulation of the transcription reaction.
In addition to the commercially available azobenzene phosphoramidite, we have also developed several advanced azobenzene derivatives.9-11 However, most of the photo-regulatory work can be achieved with the current commercial version. Since hybridization is a fundamental supramolecular property of DNA and most of the activity of DNA-based nanomachines is attributed to spontaneous hybridization, these machines can be easily converted to photo-responsive nanomachines just by adding azobenzenes into the sequence. Photo-regulated transcription and subsequent translation, i.e., photo-regulated gene expression, is also possible with a photo-responsive promoter.
References
- H. Asanuma,et al., Angew. Chem. Int. Ed. 2001, 40, 2671–2673.
- H. Asanuma, et al., Nat. Protoc. 2007, 2, 203−212.
- Y.Kamiya and H. Asanuma, Acc. Chem. Res. 2014, 47, 1663−1672
- X.G. Liang, et al., J. Am. Chem. Soc. 2003, 125, 16408–16415.
- H. Ito, et al., Org. Biomol. Chem. 2010, 8, 5519−5524.
- X.G. Liang, et al., J. Am. Chem. Soc. 2002, 124, 1877−1883.
- M. Zhou, et al., Angew. Chem., Int. Ed. 2010, 49, 2167−2170.
- M. Liu, et al., J. Am. Chem. Soc. 2003, 128, 1009–1015.
- H. Nishioka, et al., Chem. Commun. 2007, 4354−4356.
- T. Fujii, et al., , Chem. Eur. J. 2009, 15, 10092−10102.
- H. Nishioka, et al. Angew. Chem., Int. Ed. 2012, 51, 1165−1168.
Ordering Information
- Glen Report 27.11: Reversible Photo-Switching of DNA Function with Azobenzene-Tethered DNA
- Glen Report 27.12: New Products - Dithiol Serinol Phosphoramidite and 3’- Dithiol Serinol CPG
- Glen Report 27.13: Technical Brief - APA: An Alternative to AMA Deprotection
- Glen Report 27.14: Photocleavable Biotin Linker for Use in SOMAscan™
- Glen Report 27.15: PC Modifiers
- Glen Report 27.16: Technical Brief - Capping and Trityl-Protected Amino-Modifiers
- Glen Report 27.17: Technical Brief - DBCO-dT - An Unusual Case of Iodine Sensitivity

