Glen Report 16.27: New Epoch Products - 5'-Aldehyde-Modifier C2 Phosphoramidite and Gig Harbor Green™ Phosphoramidite
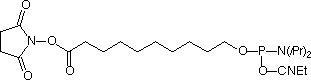
Oligonucleotide conjugation reactions are predominantly carried out using a nucleophilic group on the oligonucleotide to attach to an electrophilic group on a tag or support. This strategy presumably predominates since oligonucleotide deprotection schemes are carried out by bases, which are inherently nucleophilic. Indeed, there are many strategies to produce oligonucleotides modified with amine and thiol groups at a variety of positions. On the other hand, there are situations where researchers wish to conjugate a variety of nucleophilic groups to oligonucleotides. In this vein, convenient carboxy-modifiers have been described recently. 5'-Carboxy-Modifier C10 (1) which contains a carboxylate NHS ester (available from Glen Research) allows convenient solid-phase conjugation of amino compounds to oligonucleotides on the solid support prior to deprotection.1 Similarly, another reagent containing a protected carboxylic acid and suitable for solid-phase conjugation reactions has been described2 recently. A phosphoramidite containing an electrophilic chloroacetyl group has been described.3 In the latter case, the oligonucleotide can also be derivatized on solid phase, but the chloroacetyl group is also stable to deprotection with potassium carbonate in methanol and oligonucleotides modified with this reagent can also be used for solution-phase conjugations.
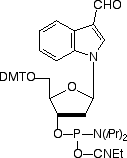
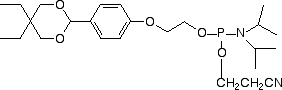
Aldehyde modifiers would be attractive electrophilic substitutions in oligo-nucleotides since they are able to react with amino groups to form a Schiff's base, with hydrazino groups to form hydrazones, and with semicarbazides to form semi-carbazones. The Schiff's base is unstable and must be reduced with sodium borohydride to form a stable linkage but hydrazones and semicarbazides are very stable linkages. Similarly to activated carboxylic acids, aldehydes are generally unstable to oligonucleotide deprotection conditions. Many strategies have used post synthetic oxidation of a glycol with sodium periodate to generate an aldehyde group. However, a recent report describes4 the use of a formylindole nucleoside analog (2) to generate an aldehyde group directly in an oligonucleotide without the need for a protecting group on the aldehyde. Our collaboration with Epoch Biosciences has allowed us to adopt the protected benzaldehyde derivative (3) as our first 5'-aldehyde modifier.5 The acetal protecting group is sufficiently hydrophobic for use in RP HPLC and cartridge purification and is readily removed after oligonucleotide synthesis under standard oligonucleotide detritylation conditions with 80% acetic acid or 2% TFA after cartridge purification.
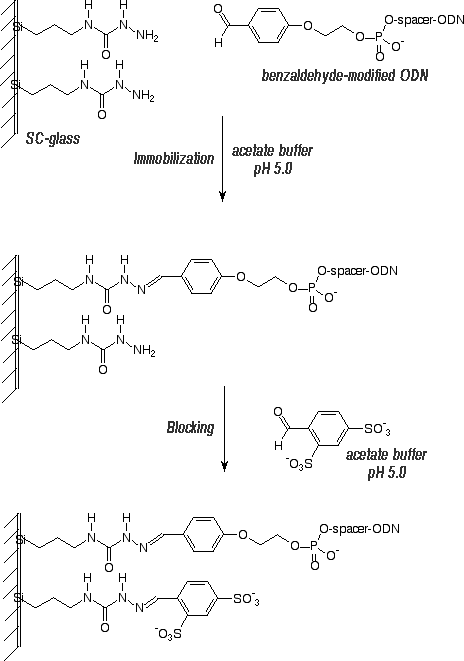
Scheme 1 illustrates the utility of this 5'-aldehyde modifier in the preparation of oligonucleotide arrays on glass slides. First, glass slides were derivatized with semicarbazidopropyltriethoxysilane, a reagent readily prepared by the reaction of isocyanopropyltriethoxysilane with hydrazine. The slides were washed, dried and cured at 110°C to produce SC-glass slides. The aldehyde-modified oligo-nucleotides were prepared with a Spacer 18 molecule between the benzaldehyde group and the oligonucleotide to help separate the oligonucleotides from the glass surface for efficient hybridization with the target. Arrays can be prepared by spotting the 5'-aldehyde-modified oligonucleotides in acetate buffer at pH 5 onto the plates and maintaining the slides in a humid environment for 3 hours at 37°C. The slides were washed to remove excess oligonucleotide and then excess semicarbazide residues on the slide were blocked with 5-formyl-1,3-benzene-disulfonic acid disodium salt. The slides were then ready for hybridization experiments.
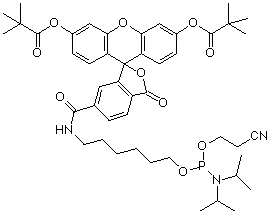
Epoch Gig Harbor Green (4) and 6-FAM (5) are based on the same fluorescein core structure. The difference between the two molecules is the linker strategy. Therefore, there should be little difference between Gig Harbor Green and FAM in terms of absorbance and emission spectra. It is well known that the fluorescence of fluorescein-based dyes depends on the degree of ionization of the phenolic groups and, therefore, is pH dependent. Gig Harbor Green is more pH dependent than FAM, which means that at pH below 7.5 fluorescence will drop slightly faster for Gig Harbor Green than for FAM. On the other hand, at higher pH such as 8.5 (PCR conditions) both dyes are completely ionized and Gig Harbor Green is 15-20% brighter than FAM.6
5'-Aldehyde-Modifier and Gig Harbor Green are offered in collaboration with Epoch Biosciences, Inc. Please read the following statement before choosing to use these products commercially.
"These Products are for research purposes only, and may not be used for commercial, clinical, diagnostic or any other use. The Products are subject to proprietary rights of Epoch Biosciences, Inc. and are made and sold under license from Epoch Biosciences, Inc. There is no implied license for commercial use with respect to the Products and a license must be obtained directly from Epoch Biosciences, Inc. with respect to any proposed commercial use of the Products. “Commercial use” includes but is not limited to the sale, lease, license or other transfer of the Products or any material derived or produced from them, the sale, lease, license or other grant of rights to use the Products or any material derived or produced from them, or the use of the Products to perform services for a fee for third parties (including contract research)."
References
- RI Hogrefe and MM Vaghefi, 2001, US Patent No. 6,320,041.
- A.V. Kachalova, D.A. Stetsenko, E.A. Romanova, V.N. Tashlitsky, M.J. Gait, and T.S. Oretskaya, Helv Chim Acta, 2002, 85, 2409-2416.
- A. Guzaev and M. Manoharan, Nucleos Nucleot, 1999, 18, 1455-1456.
- A. Okamoto, K. Tainaka, and I. Saito, Tetrahedron Lett, 2002, 43, 4581-4583.
- M.A. Podyminogin, E.A. Lukhtanov, and M.W. Reed, Nucleic Acids Res, 2001, 29, 5090-8.
- E. Lukhtanov, Epoch Biosciences, Personal Communication.

Product Information
ELITechGroup Dyes and Quenchers
Epoch Gig Harbor Green Phosphoramidite has been discontinued
- Glen Report 16.21: Thymine dimers - DNA Lesions Induced By Sunlight CIS-SYN Thymine Dimer Phosphoramidite Now Available
- Glen Report 16.22: EraGen BioSciences Technology (courtesy of EraGen Biosciences)
- Glen Report 16.23: Activators, Columns and Plates
- Glen Report 16.24: Locked Nucleic Acid (LNA™) Phosphoramidites
- Glen Report 16.25: Trimer (Codon) Phosphoramidites Simplify Library Preparation
- Glen Report 16.26: Still More New Products
- Glen Report 16.27: New Epoch Products - 5'-Aldehyde-Modifier C2 Phosphoramidite and Gig Harbor Green™ Phosphoramidite

