Glen Report 35-23: New Products BHQ-1 Phosphoramidite and BHQ-2 Phosphoramidite
Glen Research introduced many versions of the Black Hole Quencher™ (BHQ) dyes in 2002.1 Among those products are the BHQ-1 and BHQ-2 5′-Phosphoramidites, dT, and the 3′ CPG versions. In this article, we are introducing two new non-nucleoside versions: BHQ-1 and BHQ-2 Phosphoramidites. The only difference in the structure between the 5′-BHQ-1 and 2 phosphoramidites and the structure of BHQ-1 and 2 phosphoramidites is the addition of the O-4,4′-dimethoxytrityl “O-DMT” in the new versions (Figure 1). The addition of the DMT group will allow the incorporation of these modifications internally or at the 3′-end of the oligonucleotide by using one of our universal supports.
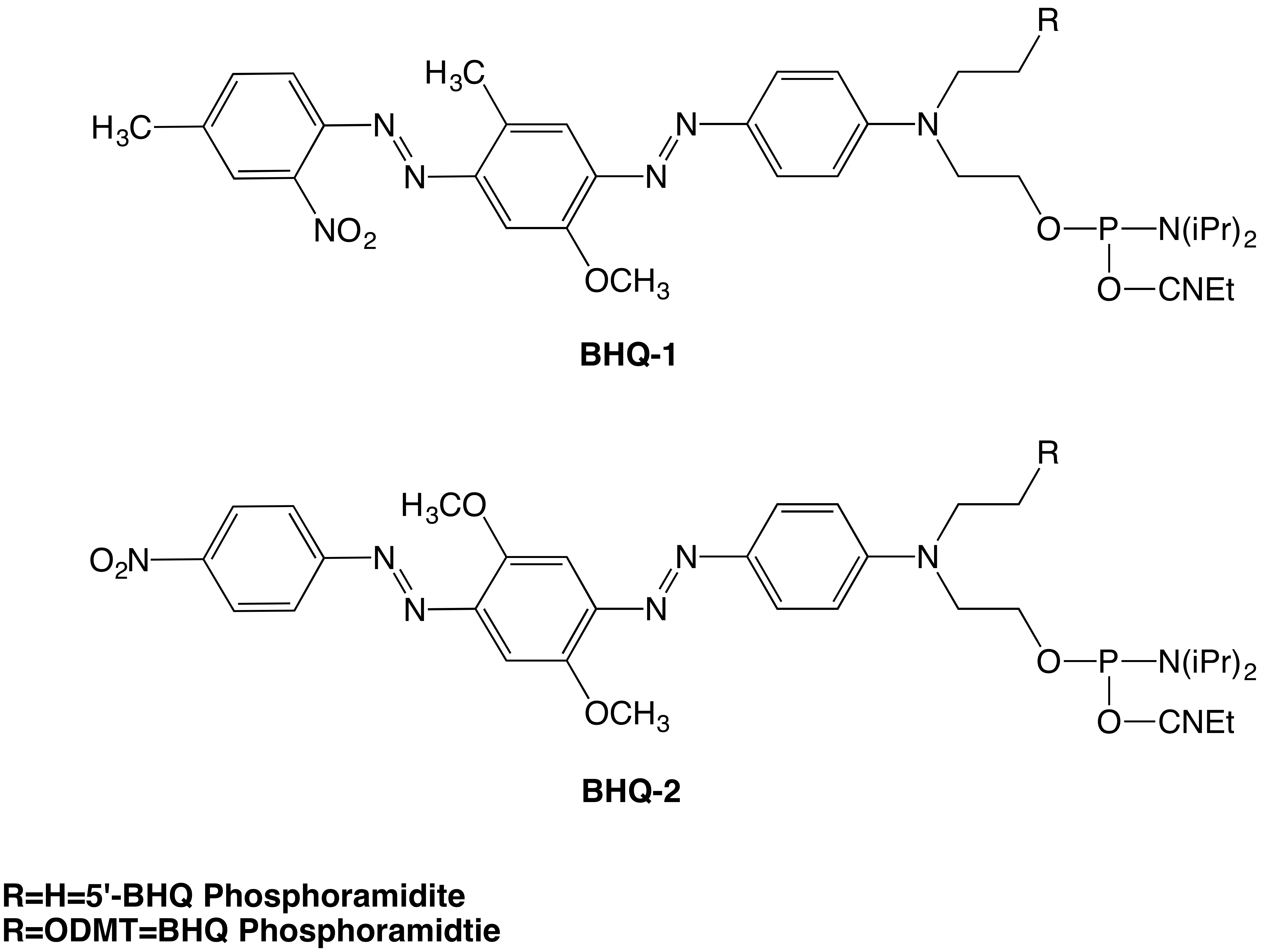
Figure 1. Structures of the BHQ-1 and BHQ-2 Phosphoramidites
Like other dark quenchers, such as Dabcyl, BHQ products consist of a polyaromatic-azo backbone (Ph-N=N-Ph). This allows them to absorb a broader range of wavelengths in the visible spectrum, resulting in the absorption of many colors. Alteration of the electron-withdrawing group (e.g., NO2) and the electron-donating group (e.g., Me) in the aromatic rings of the BHQ’s structure provides many BHQs, such as BHQ-1 and BHQ-2 with shifted spectral properties (Table 1 and Figure 2).1
| BHQ Quencher |
λmax (nm) |
E260 (L/mol•cm) |
Emax (L/mol•cm) |
| BHQ-1 | 534 | 8,000 | 34,000 |
| BHQ-2 | 579 | 8,000 | 38,000 |
Table 1. Physical properties of BHQ-1 and BHQ-2
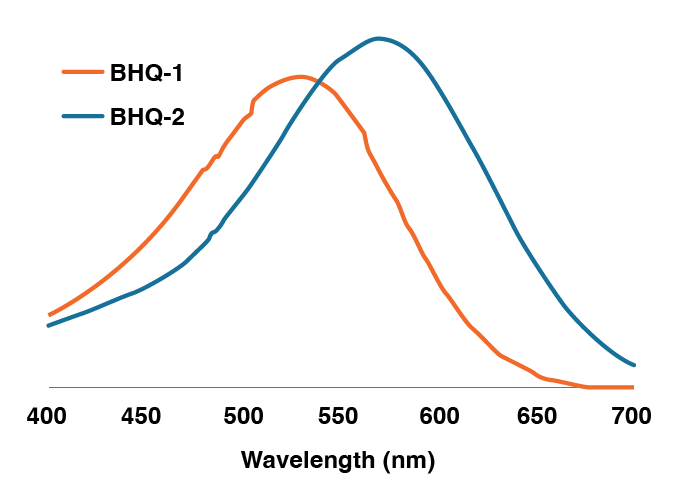
Figure 2. Visible spectra of BHQ-1 and BHQ-2
When first introduced to the market in 2000, researchers believed that BHQ probes operated only via the FRET quenching mechanism in which the excited fluorophore “donor” transfers energy to a quencher “acceptor” when they are both in close proximity (~20-100 Å). However, in 2002, Marras, Kramer, and Tyagi2 concluded that the quenching in BHQ dual-labeled probes occurs through a combination of FRET and static quenching mechanisms. The affinity of the fluorophore for BHQs in the probe leads to the two binding each other, producing a non-fluorescent intramolecular dimer in the static quenching mechanism.
BHQs Applications
In addition to their broad absorption spectra, BHQs have large extinction coefficients, excellent coupling efficiencies, are compatible with ammonium hydroxide deprotection, and are completely non-fluorescent quenchers. These factors contributed to the popularity of BHQs in building dual probes with several fluorophores. As a result, BHQs were used extensively during the COVID-19 pandemic. Most of the World Health Organization’s COVID-19 testing protocols used BHQs, as they became favorable quenchers for quantitative real-time PCR (qPCR).3 Moreover, most of the manufacturers in the molecular diagnostic field use BHQ in their assays.
BHQs have not only played an essential role during the COVID-19 pandemic in diagnosing patients with COVID-19, but they are now playing another important role in the early detection of disease outbreaks. Non-fluorescent quenchers, such as BHQs, are recommended in the recent Minimum Information for Publication of Quantitative Real-Time PCR Experiments (MIQE) guidelines for hydrolysis probes used in Digital PCR (dPCR).4 Currently, the BHQs are used with dPCR to check the concentration of the SARS-CoV-2 in wastewater.
Use of BHQ-1 and BHQ-2 Phosphoramidites
Much like the 5′-BHQ-1 and 2 phosphoramidites, no changes are needed from the standard coupling method recommended by the synthesizer manufacturer. The deprotection also follows the standard method recommended by synthesizer manufacturers, except that the use of methylamine in ultrafast deprotection protocols will result in degradation to the dye and is not recommended in the case of the BHQ-2 phosphoramidite. The optimal deprotection conditions for probes that contain BHQ-1 or BHQ-2 and other dyes in the dual-labeled probes can be found in our recent Glen Report (34.2).5
“Black Hole Quencher” is a trademark of Biosearch Technologies, Inc., Novato, CA.
References
- The Glen Report, 2004, 17.1, 4-6.
- S.A. Marras, F.R. Kramer, and S. Tyagi, Nucleic Acids Res, 2002, 30, e122.
- S.A. Bustin, and T. Nolan, Int J Mol Sci, 2020, 21, 3004-3012.
- dMIQE Group and J.F. Huggett, Clin Chem, 2020, 66, 1012-1029.
- The Glen Report, 2022, 34.2, 1-2.
Product Information
- Glen Report 35-21: CleanCap® M6
- Glen Report 35-22: New Product 2′-OMe-N6-Me-A-CE (m6Am) Phosphoramidite
- Glen Report 35-23: New Products BHQ-1 Phosphoramidite and BHQ-2 Phosphoramidite
- Glen Report 35-24: Application Note Degenerate Oligonucleotides
- Glen Report 35-25: Product Review DBCO
- Glen Report 35-26: Technical Snippets

