Glen Report 26.27: Technical Brief – Use of Click Chemistry for Possible Crosslinking, Click Synthesis of a Potentially Useful azido support and New Click Product - Cyanine 7 Azide
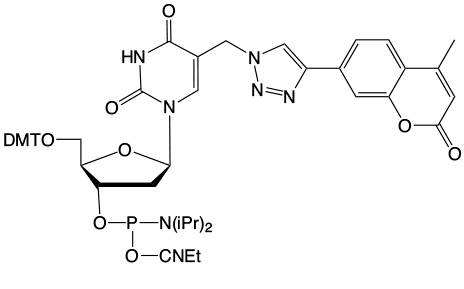
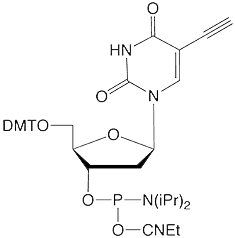
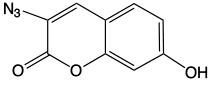
Possible Crosslink
As our regular readers know, Glen Research has a long term interest in oligonucleotide interstrand crosslinking. During the enforced absence of cnvK, we continue to evaluate new methods that may allow a practical approach to crosslinking.
A recent publication1 from Professor Peng and his group at the University of Wisconsin, Milwaukee, along with researchers from the University of California, Riverside, caught our attention. Using a synthetic approach involving click chemistry, a coumarin containing thymidine analogue, (1) in Figure 1, was prepared and incorporated into oligonucleotides using standard phosphoramidite chemistry. Interstrand crosslinking was achieved by irradiation using UV light at 350nm. The authors demonstrated that crosslinking occurs preferentially with dT on the opposite strand but also occurs at lower levels with dC and dA. The photo-induced crosslink with pyrimidines can be reversed with UV light at 254nm but is irreversible with purines.

Coumarin-Thymidine Analogue Crosslinked with Thymidine
While coumarin is not fluorescent (as we have noted in the past in relation to our coumarin azide) the triazole product of the click reaction is fluorescent. Although the authors describe a slightly different coumarin structure, the coumarin-thymidine analogue is fluorescent until a crosslink is formed, which shows that the conjugation system of the coumarin has been disrupted. This behavior indicates that the crosslinking occurs via a [2+2] cycloaddition with a double bond on the base opposite. The proposed crosslink with thymidine is shown in Figure 2.


The authors also demonstrated that the interstrand crosslink was photoswitchable over six cycles of radiation at 350nm and 254nm. This may be the first observation of photoswitching of interstrand oligonucleotide crosslinks induced by a modified pyrimidine analogue.
This elegant work piqued our interest since we offer 5-ethynyl-dU, (2) in Figure 1, as a potential synthon for preparing possible crosslinking intermediates following a click reaction. As shown in Figure 3, the click reaction with our coumarin azide, (3) in Figure 1, forms an intermediate which also may be interesting for potential crosslinking. However, the location of the double bond which would be a partner in the [2+2] cycloaddition is close to the triazole ring and that may not be optimal in comparison to Professor Peng's structure.
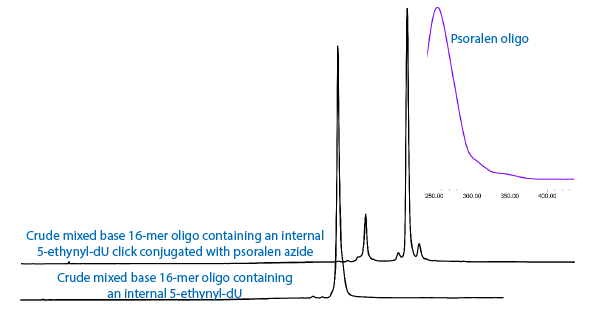
A second option may be psoralen azide, (4) in Figure 1. The click reaction of psoralen azide with ethynyl-dU forms a potentially more interesting intermediate, as shown in Figure 3. In this case, the well known crosslinking behavior of psoralen with an adjacent thymidine residue may be indicated.
At this point, we simply demonstrate that both coumarin and psoralen azides form triazole intermediates with 5-ethynyl-dU, as shown in the chromatograms in Figure 4. We also introduce psoralen azide to our growing selection of azido tags.
Potential Azide Support
One of our customers inquired whether it would be possible to prepare an azide solid support for oligonucleotide synthesis for later reaction to an alkyne containing tag. A reference from the Morvan group2 certainly indicates that an azide solid support can indeed survive the conditions of oligonucleotide synthesis and can be used to click to a suitable alkyne containing product. So we set out to determine if an azide support could be simply prepared using our existing products. Our strategy is outlined in Figure 5.

We used our 3'-Amino-Modifier C7 CPG (20-2958) and removed the Fmoc protecting group with 20% piperidine in DMF. It was then straightforward to conjugate the free alkylamine on the support to our azidobutyrate NHS ester to generate the desired azide support.

After regular oligonucleotide synthesis and deprotection, the purified oligonucleotide was subjected to a click reaction with a dabsyl alkyne to confirm the presence of an active azide at the 3' terminus. The results, shown in Figure 6, confirm that the dabsyl dye was click conjugated and that the azide support was successfully prepared.
References
- M.M. Haque, H.B. Sun, S. Liu, Y.S. Wang, and X.H. Peng, Angewandte Chemie-International Edition, 2014, 53, 7001-7005.
- G. Pourceau, A. Meyer, J.J. Vasseur, and F. Morvan, J Org Chem, 2009, 74, 6837-6842.
See Also: New Product - Disulfo-Cyanine 7 Azide
Product Information
Disulfo-Cyanine 7 Azide (50-2010) has been discontinued.
Psoralen Azide (50-2009)
Coumarin Azide (50-2004)
- Glen Report 26.21: Reversible M6A RNA Modification
- Glen Report 26.22: New Product - N6-Me-A -Reversible M6A RNA Modification
- Glen Report 26.23: New Product - 5'-Carboxy-Modifier C5
- Glen Report 26.24: DNA-Mediated Charge Transport: A Novel Property of DNA with Diverse Applications
- Glen Report 26.25: New Product - Methylene Blue NHS Ester
- Glen Report 26.26: Technical Brief - Which 3'-Amino-Modifier?
- Glen Report 26.27: Technical Brief – Use of Click Chemistry for Possible Crosslinking, Click Synthesis of a Potentially Useful azido support and New Click Product - Cyanine 7 Azide
- Glen Report 26.28: New Product - Disulfo-Cyanine 7 Azide

