Glen Report 25.14: Novel Reagents Utilizing a Serinol Scaffold for Labeling Synthetic Oligonucleotides
U.S. Patent No. 8,394,948
Novel reagents utilizing a serinol scaffold for labeling synthetic oligonucleotides
We are pleased to announce that the above titled U.S. Patent was approved and issued March 12, 2013. The inventors are Paul Nelson, Hugh Mackie and Andrew Murphy and the patent has been assigned to Glen Research Corporation and Nelson Biotechnologies, Inc.
The abstract states the following:
“Novel CE-phosphoramidites and CPG reagents have been synthesized from a serinol backbone. These reagents are useful to introduce functional groups or directly label oligonucleotides. The versatile serinol scaffold allows for labeling at any position (5’ or 3’ termini, or any internal position) during automated DNA synthesis. Multiple labels or functional groups can be achieved by repetitive coupling cycles. Optimal spacer arms and protected label moieties have been specially designed. Further, the natural 3-carbon atom internucleotide phosphate distance is retained when inserted internally.”
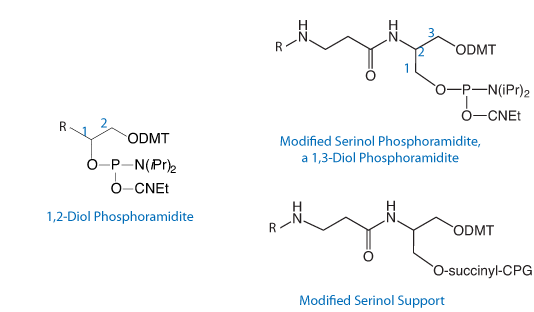
Our serinol-based line of products for modification and labeling has been available for some time and the products have proved to be very popular in a variety of research applications. Although these products are now covered by U.S. Patent, there will only be the usual limitation in the use of these products. All of Glen Research’s products are primarily for research use only and this applies to the serinol line regardless of whether oligos containing them are prepared by the end user or a core facility or a custom oligo company. Clearly, we wish to encourage the use of these valuable products. It is also our intent that IVD companies, for example, would be able to use these products to overcome any IP issues associated with alternative products for modification and labeling.
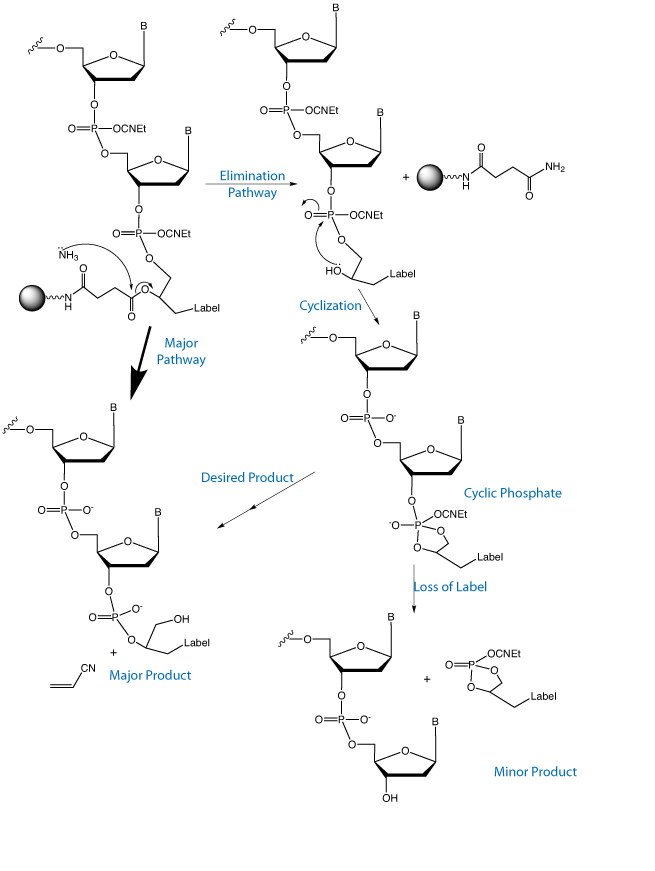
The significance of this product line is described in the background of the invention.
Current methods to introduce chemical modifications into oligonucleotides have limitations. Many non-nucleosidic phosphoramidite reagents are limited to single modifications at the 5’ terminus, thus terminating chain elongation at the point of introduction. Those designed for multiple incorporations, such as 1,2-diol backbone phosphoramidite reagents, also suffer some drawbacks. The internucleotide distance, when incorporated internally, results in a constricted internucleotide phosphate distance one carbon atom shorter than the natural DNA structure. Further, the 1,2-diol backbone can participate in a dephosphorylation reaction due to a highly favorable 5-membered cyclic phosphate intermediate, resulting in cleavage of the label. Other reagents suffer from poor design in protecting label moieties. For example, some biotin phosphoramidite reagents do not protect its urea moiety. Hence, phosphoramidites can react at this active position of biotin in unwanted side reactions.
The 1,3 diol reagents of Nelson et al have proven to be superior, overcoming the above disadvantages, albeit, improved protection of label moieties has not been addressed. The subject of this invention builds upon the advantages of the 1,3 diol reagents by utilizing a serinol backbone. This backbone is versatile, readily available, and allows for convenient preparation of reagents. The purpose of this invention is to overcome the disadvantages encountered in the prior art by providing improved reagents to directly modify or label oligonucleotides via automated DNA synthesis.
The general structure of our serinol products is shown in Figure 1 and the wide variety of products we offer is shown in the Ordering Information table on the right.
In Figure 2, we illustrate the side reaction inherent in 1,2-diol based products that leads to significant label loss during deprotection. This reaction is competitive with simple hydrolysis of the protecting groups and leads to some loss of label. Fortunately, the elimination reaction is virtually non-existent in the 1,3-diol backbone since the cyclic intermediate would be a 6-membered ring which is not favored for a cyclic phosphate intermediate.
Glen Research is pleased to offer to our research customers these stable and efficient reagents for modifying and labeling oligonucleotides.
Product Information
- Glen Report 25.11: New Product - (5'S)-5',8-Cyclo-dG - DNA Damage and Repair
- Glen Report 25.12: Antisense Trimer Phosphoramidites - Update
- Glen Report 25.13: Technical Brief - Side Reaction of Fluorescein during Deprotection with Methylamine
- Glen Report 25.14: Novel Reagents Utilizing a Serinol Scaffold for Labeling Synthetic Oligonucleotides
- Glen Report 25.15: Technical Brief - Aldehyde and Aminooxy Conjugations
- Glen Report 25.16: Technical Brief - Amino-Modifiers and Summer Shipping

