Glen Report 25.13: Technical Brief - Side Reaction of Fluorescein during Deprotection with Methylamine
Fluorescein in its most popular 6-carboxy-fluorescein (FAM) form is one of the most ubiquitous fluorescent dyes used to label DNA. With its high molar extinction coefficient, high quantum yield of fluorescence and good stability toward DNA synthesis and deprotection chemistries, FAM continues to be one of the most popular fluorophores on the market.
However, there is a bit of a chemical mystery associated with it - under certain conditions, a late-eluting peak is observed in oligos that exhibits no absorbance in the visible spectrum. We found that the impurity appeared when using AMA (ammonium hydroxide/40% aqueous methylamine 1:1 v/v) to deprotect a FAM-labelled oligo and was present whether the oligo was deprotected at room temperature or at 65 °C. Figure 1a contains the RP HPLC of FAM coupled to a T6 oligo when deprotected in AMA for 10 minutes at 65 °C, which shows the later eluting impurity at a concentration of around 5%.
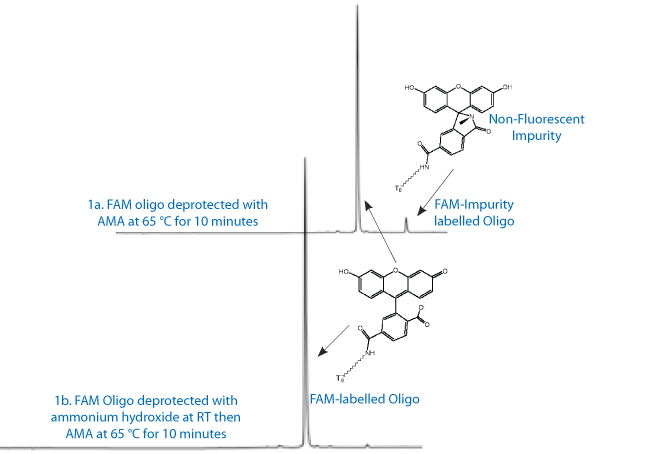
Two other observations should be noted. The first is that FAM is perfectly stable when deprotected in concentrated ammonium hydroxide even for 17 hours at 55 °C - a chemical stability which is rare for fluorophores. The second is that the relative amount of this later eluting impurity was the same whether the oligo is deprotected in AMA for 10 minutes or for 60 minutes at 65 °C. So, it appears that the FAM is stable to the AMA solution - but only after the pivaloyl protecting groups of the 3' and 6' hydroxyls have been removed, at which point the FAM is no longer susceptible to degradation by AMA.
To identify the impurity, a FAM-labelled oligo deprotected in AMA was analyzed by Electrospray MS. The FAM side product had a molecular weight (mw) of +13 Da. The structure that is consistent with the mw of the impurity as well as its lack of absorbance in the visible spectrum is shown in Figure 1a. We propose that a nucleophilic attack by the methylamine may occur at position C1 which ultimately leads to a non-hydrolyzable amide. A proposed mechanism is shown in Figure 2.
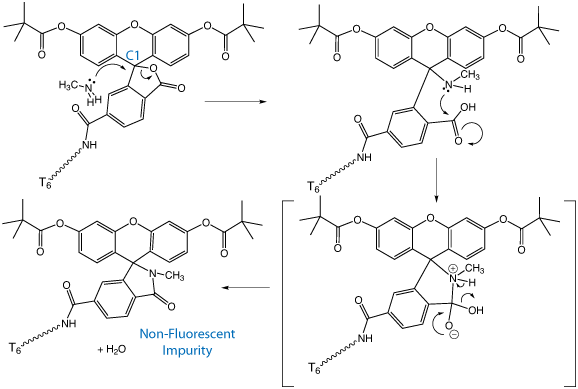
Why the analogous reaction does not occur with ammonia is not clear. We can only surmise that, with the greater nucleophilicity of methylamine, the relative rate of nucleophilic attack at the C1 of the spiro-carbon of the cyclic lactone is significant compared to the rate of hydrolysis of the pivaloyl ester. With ammonia, the rate of nucleophilic attack at the C1 must be low, making the amount of the non-fluorescent lactam insignificant.
This difference can be used to advantage, though, by first treating the protected FAM-labelled oligo with ammonium hydroxide while it is still on the support. Once the yellow-green color of the fluorescein is evident, which indicates the the pivaloyl groups have been removed, the methylamine solution can be safely added. Shown in Figure 1b is the chromatogram of FAM-T6 from the same synthesis as shown in Figure 1a. However, this time the oligo was first briefly deprotected in ammonium hydroxide and then the 40% methylamine was added to complete the deprotection. Note the rate of removal of the pivaloyl protecting groups by ammonia will depend upon the length of the oligo and the location of the FAM - i.e., whether it is 3' or 5'.
Product Information
- Glen Report 25.11: New Product - (5'S)-5',8-Cyclo-dG - DNA Damage and Repair
- Glen Report 25.12: Antisense Trimer Phosphoramidites - Update
- Glen Report 25.13: Technical Brief - Side Reaction of Fluorescein during Deprotection with Methylamine
- Glen Report 25.14: Novel Reagents Utilizing a Serinol Scaffold for Labeling Synthetic Oligonucleotides
- Glen Report 25.15: Technical Brief - Aldehyde and Aminooxy Conjugations
- Glen Report 25.16: Technical Brief - Amino-Modifiers and Summer Shipping

