Glen Report 24.22: New Product - DBCO-dT for Copper-Free Click Chemistry
Introduction
The copper(I) catalyzed [3+2] azide-alkyne cycloaddition (CuAAC) is the most prominent example of a group of reactions known as click reactions, as shown in Figure 1. Glen Research has been active in supporting Click Chemistry for several years and, in this newsletter, we highlight an excellent example of click ligation to generate a biocompatible internucleotide linkage (Page 1), a simplified kit-based click system (Page 8), and some new products for CuAAC Click Chemistry (Page 5).
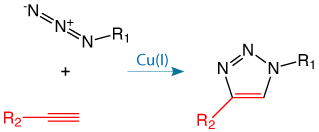
Copper-free click has some advantages over CuAAC, especially in situations where users do not perform click reactions regularly and are looking for a simplified alternative to CuAAC. Cyclooctyne is the smallest cyclic octyne that can be isolated. Because of the severe deformation of the alkyne from its desired linear geometry, cyclooctynes are highly reactive towards azides without the need for copper catalysis.
Copper-Free Click
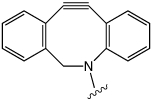
From the variety of cyclooctyne-based copper-free click reagents so far described, we chose to offer compounds based on the dibenzocyclooctyl (DBCO) structure, shown in Figure 2.1-3 DBCO products exhibit the following desirable properties:
- Simple to use
- Stable in solution on the synthesizer
- Stable to ammonium hydroxide and AMA
- Excellent click performance in 17 hours or less at room temperature
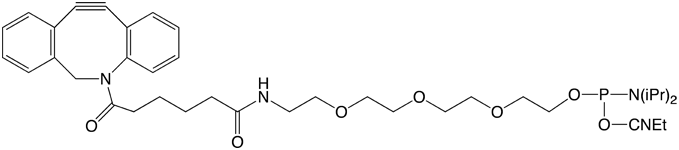
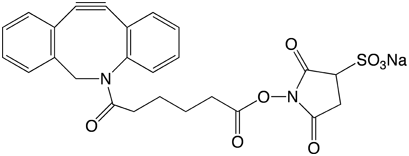
For 5'-modification, we chose to use 5’-DBCO-TEG Phosphoramidite (1), in which the very hydrophobic DBCO moiety is separated from the phosphoramidite and subsequent oligo with a triethyleneglycol (TEG) spacer. In addition, we chose to offer a soluble DBCO-sulfo-NHS ester sodium salt (2) for post-synthesis conjugation reactions with amino-modified oligonucleotides and proteins.
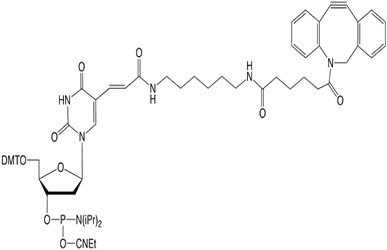
In addition to these DBCO-based products, we now offer DBCO-dT-CE Phosphoramidite (3) for inserting a DBCO group at any position within the oligonucleotide. This type of dT analogue has proved to be popular in the past since the tag is projected into the major groove of duplex DNA where it does not disrupt the DNA duplex while being readily accessible for further reaction.
Synthesis and Deprotection
A coupling time of 12 minutes was found to be optimal for DBCO-dT (3). It was found that DBCO-modified oligos were stable to deprotection with ammonium hydroxide for 2 hours at 65°C or overnight at room temperature, which would allow the use of regular phosphoramidites, including dmf-dG but not ibu-dG. Deprotection with AMA for 2 hours at room temperature showed only slight degradation of the cyclooctyne, making the modification compatible with ibu-dG if Ac-dC is used. DBCO-modified oligos are also compatible with UltraMild deprotection conditions.
References:
- P. van Delft, et al., Org Lett, 2010, 12, 5486-9.
- P. van Delft, et al., Synthesis-Stuttgart, 2011, 2724-2732.
- I.S. Marks, et al., Bioconjugate Chemistry, 2011, 22, 1259-1263.
Product Information
5'-DBCO-TEG Phosphoramidite (10-1941)
DBCO-sulfo-NHS Ester (50-1941)
DBCO-dT-CE Phosphoramidite (10-1539)
- Glen Report 24.21: Click DNA and RNA Ligation – A Biocompatible Triazole Linkage
- Glen Report 24.22: New Product - DBCO-dT for Copper-Free Click Chemistry
- Glen Report 24.23: Antisense Trimer Phosphoramidites - A New Method for Mutagenesis Spanning Entire Genes
- Glen Report 24.24: New Product - Alkyne-Modifier Serinol Phosphoramidite
- Glen Report 24.25: Technical Brief - Reagents for 5’-Labeling of microRNAs
- Glen Report 24.26: Advances in Copper(I)-Catalyzed Azide-Alkyne Cycloaddition (CuAAC)
- Glen Report 24.27: Introducing Oligo-Click Kits
- Glen Report 24.28: Technical Brief: FRETmatrix – A Method for Simulation and Analysis of FRET in Oligos
- Glen Report 24.29: Technical Brief: Which 5'-Amino-Modifier?
- Glen Report 24.210: Technical Brief - Improved Conditions for Deprotecting 5-Formyl-dC
- Glen Report 24.211: New Products: Universal HybridCPG™ Solid Supports