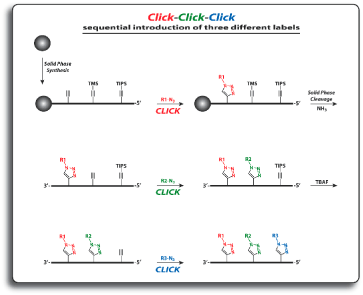
For the introduction of three different labels, three alkyne nucleosides, one with no alkyne protection, the second with TIPS protection, and the third with TMS protection, are introduced into oligonucleotides. The first click reaction is performed directly on the resin. The singly modified oligonucleotide is subsequently cleaved from the support with concomitant cleavage of the TMS group and retention of the TIPS protecting group. The second click reaction is performed in solution. Precipitation of the doubly modified oligonucleotide, cleavage of the TIPS group with TBAF, and a subsequent third click reaction in solution furnishes the desired triply modified oligonucleotides in excellent overall yields.
CuAAC requires the direct use of Cu(I) such as cuprous bromide (CuBr) or a source for Cu(I) such as the combination of Cu(II) salts and a reducing agent (e.g., CuSO4 and sodium ascorbate). The presence of a Cu(I)-stabilizing ligand, such as TBTA, increases the efficacy and decreases the reaction time of the CuAAC. For optimal reaction results, solutions must be freshly prepared and eventually degassed prior to use. Solubility of the ligand TBTA in diluted aqueous solutions may be an issue as well. Although this is not burdensome for regular use, occasional users can find the process troublesome.
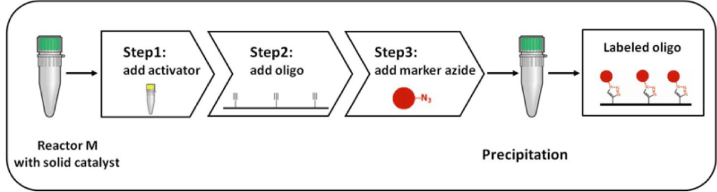
To overcome these limitations, baseclick now offers a simple solution: the Oligo-Click Kits. These kits contain an air-stable, insoluble Cu(I) source in pellet form in a pre-loaded and ready-to-use vial. Within the kit, the TBTA ligand is replaced by an activator which is compatible with both aqueous and organic solvents. This innovative combination of catalyst and ligand/activator results in a much easier labeling work-flow including only three simple steps. The preparation of the oligonucleotide labeling via CuAAC now requires only a minimal hands-on time of a few minutes or even less and can be carried out in air without any additional precautions (Figure 3).
The CuAAC reaction time depends on many factors such as label size, oligonucleotide sequence, number of alkynes and other modifications within the sequence, reaction temperature, concentration of the oligonucleotide, as well as the azide in the reaction mixture. Typically, a complete conversion of a single labeled oligonucleotide using the Oligo-Click Kit is achieved in less than 1 hour when operating at 45 °C with an alkyne / azide ratio of 1:2. Lower reaction temperatures (e.g., room temperature or even 4 °C) can be efficiently applied as well in combination with longer reaction times (4 hour and overnight, respectively). The efficacy of the CuAAC reaction remains very high and in some cases even superior to the efficacy of the CuBr / TBTA system.
Labeling of oligonucleotides containing more than two alkynes normally requires the use of a larger amount of azide – up to an alkyne / azide ratio of 1:25 – as reported in the table within the kit user manual.
After the reaction, the labeling mixture is simply transferred into a new vial and the solid catalyst is discarded. No filtration is needed during this step due to the size of the catalyst pellets. Further processing of the reaction may include a precipitation step (e.g., ethanol precipitation), which removes excess of label-azide, activator and eventually organic solvents used to dissolve the azide. Thus, labeled oligonucleotides can be recovered in near quantitative yields.
Oligo-Click Kits are now available in two different sizes from baseclick:
The Oligo-Click Reload series (S and M) provides one vial containing the activator (yellow-capped vial) along with the reactor (green-capped vial) containing the catalyst in pellet form.
Azides are available in red-capped vials within the following Oligo-Click kits:
Figure 4 shows an example of RP-HPLC and MALDI-mass spectrum measured directly after the click reaction. In this case, an oligonucleotide containing two internal alkynes was labeled with ATTO425-azide using the Oligo-Click M Kit followed by a simple ethanol precipitation step without further purification.
The results show the outstanding efficiency of the Oligo-Click Kit and of CuAAC in general.

It is possible to construct highly strained ring systems containing alkyne groups that will allow Click reactions to occur without the need for a copper catalyst. However, these Cu-free Click reagents tend to be simple 5'-modifiers or dT derivatives. The catalog of alkyne derivatives for CuAAC is substantially greater. At this point, no Cu-free options are available for attaching more than one type of label or tag to oligonucleotides.
Moreover, the amount of Cu ions during the CuAAC using the Oligo-Click Kits generally does not exceed 100 ng/µL (colorimetric complexation detection). This value drops to 50 ng/µL after a simple precipitation step (e.g., EtOH-precipitation) and it is further reduced to 5-10 ng/µL after RP HPLC purification. Gel purification or ion-exchange filtrations abate the Cu ions content below the detection limit of 0.01 ng/µL.
The easy-to-use copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) reaction with its outstanding selectivity is in an excellent position to take over as the state-of-the-art methodology to label and modify DNA and nucleic acids in general. With baseclick's addition of the Oligo-Click Kits many factors have been radically improved such as the stability of the reagents, their solubility and the comfortable handling of CuAAC, thus providing a powerful labeling tool for chemistry and biology labs.
The following patents have been granted:
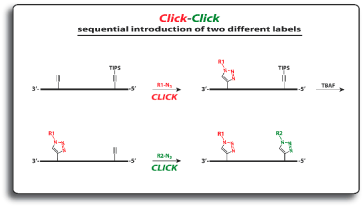
Glen Research has collaborated with baseclick for several years on this product line. We have been offering the dC and dT analogues, shown in Figure 2, for simple Click conjugations as well as sequential labeling with up to three separate azides.
We are delighted to be able to offer Oligo-Click Kits to our research customers. Our most popular scale of synthesis is 200 nmoles, so we are offering Oligo-Kit M. This kit has sufficient reagents for conjugating up to nine alkyne-containing oligonucleotides on a 100 nmole scale or a single oligonucleotide on a 1 µmole scale. The user must supply the azide and a solvent such as DMSO for dissolving the azide.
We are also offering kits for biotin, fluorescein and TAMRA labeling. Each kit has sufficient reagents for conjugating up to seven alkyne-containing oligonucleotides on a 100 nmole scale or a single oligonucleotide on a 1 µmole scale. Each kit contains all of the ingredients necessary, including the azide and DMSO solvent.
Our experience with these kits indicates that they will be of enormous help to customers carrying out click conjugation reactions on an occasional basis.