Glen Report 21.25: tC and tCo: New Tricyclic Fluorescent Cytidine Analogues with a very Bright Future
Glen Research has offered fluorescent nucleosides for a long time: 2-Aminopurine deoxynucleoside and Etheno-dA have been in our catalog for more than 15 years. More recently, we have become interested in the potential for dC analogues to offer interesting fluorescence properties while maintaining excellent base pairing behavior with dG. In partnership with Berry and Associates, we introduced pyrrolo-dC1, which has intriguing properties that allow its exact position in a duplex to be determined. And, recently, we have pointed out the fluorescence properties of AP-dC (G-Clamp).2
Now we are happy to introduce the tricyclic fluorescent nucleoside analogues, 1,3-diaza-2-oxophenothiazine, tC, and 1,3-diaza-2-oxophenoxazine, tCo. Tricyclic dC base analogues have been shown to base pair faithfully with dG with virtually no disruption of the normal duplex structure.3-5 This means that the stability of the DNA duplex is not compromised as compared to the control regardless of DNA sequence.
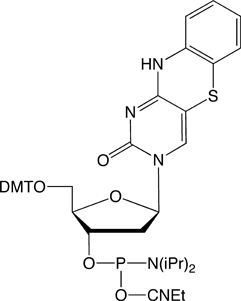
1,3-diaza-2-oxophenothiazine (tC)
Wilhelmsson and coworkers have demonstrated that tC is unique in the group of fluorescent base analogues that mimic cytosine.5 As might be predicted, the structure of duplex DNA containing tC retains the native B-form. Melting studies on duplexes containing tC have demonstrated that it base pairs strongly with G and the melting temperatures of duplexes are increased by 2.7° over the equivalent C-containing duplex.
tC is strongly fluorescent with an absorption maximum at 385nm, which is far outside the wavelength of DNA absoption. Its emission maximum is at 500nm.4
Of great significance is the fact that the fluorescence quantum yield of tC is essentially unchanged between single stranded and double stranded DNA. The average quantum yield was 0.21 for single stranded DNA and 0.19 for duplex DNA. Also, the fluorescence characteristics of tC were not sensitive to neighboring base combinations.4
This combination of characteristics make tC one of the most interesting fluorescent bases so far evaluated.
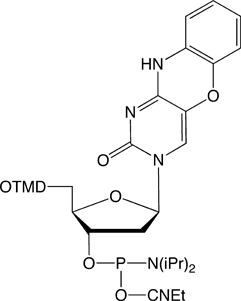
1,3-diaza-2-oxophenoxazine (tCo)
Like its sulfur-containing cousin, tCo is also a very bright fluorophore. It has been reported to be 25-50 times brighter than 2-aminopurine.3 The reason for this is twofold. First, tCo has a large extinction coefficient with an E360 of 9,000 L/mol.cm. Second, unlike other nucleoside analogs, tCo is not severely quenched when in double-stranded DNA. Peter Sandin and colleagues did an exhaustive study of neighboring base effects in single- and double-stranded oligos, determining the quantum yield of fluorescence (QY) for all permutations of bases immediately 5' and 3' to tCo when the oligonucleotide is single versus double-stranded.3 They found that on average, the QY is 0.30 for single-stranded oligos and a quite respectable 0.21 when double-stranded, making tCo the brightest fluorescent nucleoside analogue in double-stranded DNA reported so far.
Spectral Characteristics
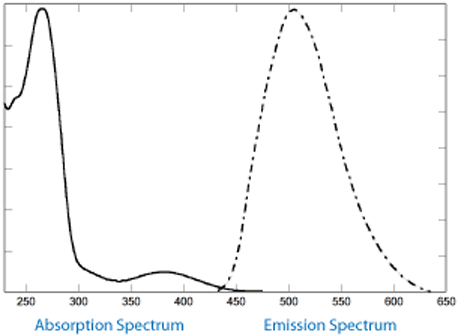
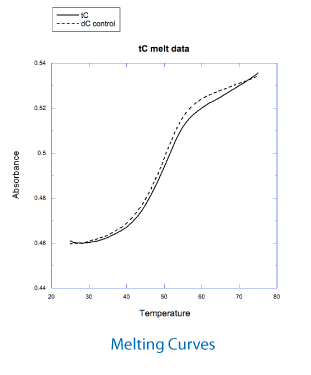
Figure 2 shows the absorption and emission spectra of the oligo 5'-ATC GXT CAT GAT G-3', where X is tC, as determined in house. The absorption maximum was found to be at 392nm with no shift in wavelength in duplex DNA. The emission maximum was found to be at 506nm. Figure 2 illustrates the melting curves for the tC oligo as well as the control where X = dC. We found that the Tm of this oligo was raised by 1.0° relative to the control oligo containing dC.
Figure 3 shows the absorption and emission spectra of the oligo 5'-ATC GXT CAT GAT G-3', where X is tCo, as determined in house. The absorption maximum was found to be at 360nm, shifting to 365nm in duplex DNA. The emission maximum was found to be at 465nm. Figure 3 also shows the melting curves for the tCo oligo, as well as the control where X = dC. We found that the Tm of this oligo was raised by 0.5° relative to the control oligo containing dC.


Synthesis and Deprotection
tC- and tCo-CE Phosphoramidites couple optimally with a reaction time of 3 minutes. Deprotection can be carried out using ammonium hydroxide at room temperature (to avoid minor degradation at elevated temperatures), AMA at 65°C or potassium carbonate in methanol, as required for deprotection of the nucleobases.
We are happy to offer tC as a very bright and stable probe of DNA structure and tCo as the brightest and most promising internal fluorescent probe for DNA to date. In combination, they offer great potential for the analysis of DNA structure and function. We would also like to thank Marcus Wilhelmsson for his help in preparing this article.
References
- D.A. Berry, et al., Tetrahedron Lett, 2004, 45, 2457-2461.
- The Glen Report, 2007, 19, 8-9.
- P. Sandin, et al., Nucleic Acids Res., 2008, 36, 157-167.
- P. Sandin, et al., Nucleic Acids Res., 2005, 33, 5019-5025.
- K.C. Engman, et al., Nucleic Acids Res., 2004, 32, 5087-5095.
Product Information
tC-CE Phosphoramidite (10-1516)
tC°-CE Phosphoramidite (10-1517)
- Glen Report 21.21: 5-Hydroxymethyl-dC: A New Actor in the Field of Epigenetics
- Glen Report 21.22: Purification of CleanAmp™ DNA Oligonucleotides (DMT-ON)
- Glen Report 21.23: A 3'-Cap for Improved Target Affinity and Specificity
- Glen Report 21.24: Metallobase Nucleic Acid Modification
- Glen Report 21.25: tC and tCo: New Tricyclic Fluorescent Cytidine Analogues with a very Bright Future
- Glen Report 21.26: Non-Aqueous Oxidation for PACE Chemistry
- Glen Report 21.27: New Product - Azobenzene Phosphoramidite for the Introduction of Photo-regulated Functions in DNA
- Glen Report 21.28: Deprotection - Volume 3 - Dye-Containing Oligonucleotides
- Glen Report 21.29: New Products - Glen UnySupport™ Frits
- Glen Report 21.210: New products: Glen-Pak™ DNA Cartridge 3g and DNA 30mg 96-Well Plates
- Glen Report 21.211: Technical Brief – Synthesis of Long Oligonucleotides

