Glen Report 14.16: Product Update - How Are Methyl Triester Linkages Prepared?
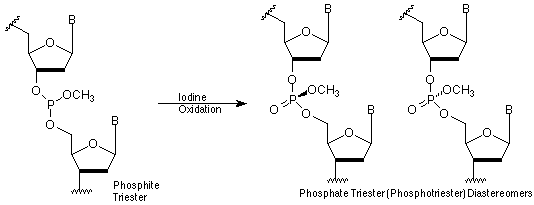
For many years, Glen Research has supplied methyl phosphoramidites in addition to ß-cyanoethyl (CE) phosphoramidites for the few situations where the more labile cyanoethyl group is not an advantage. Some of our customers, probably remembering that the methyl group was removed specifically with thiophenol, have tried to use these monomers to prepare the interesting, uncharged, and nuclease-resistant methyl phosphotriester linkage. Unfortunately, this linkage is labile to ammonium hydroxide and the regular phosphodiester linkage is formed (along with a small amount of chain scission).
Now we offer UltraMild methyl phosphoramidites! Oligos produced from these can be deprotected with potassium carbonate in methanol to produce methyl phosphotriester linkages. Since these linkages are diastereomeric and uncharged, the oligos may be hard to handle. Instead, it is likely that chimeras will be produced using these monomers along with the regular UltraMild CE phosphoramidites.
Product Information
Me Phosphoramidites have been discontinued
- Glen Report 14.11: Novel Universal Support Features Rapid Amide-Assisted Dephosphorylation
- Glen Report 14.12: A New Simplified 3'-Amino-Modifier CPG
- Glen Report 14.13: Preparation of Oligonucleotides Containing Abasic Sites
- Glen Report 14.14: PC Biotin and Related Photocleavable Modifiers
- Glen Report 14.15: New OPeC™ Reagents for Synthesis by Native Ligation of Oligonucleotide-Peptide Conjugates
- Glen Report 14.16: Product Update - How Are Methyl Triester Linkages Prepared?

