Glen Report 29.14: N-Acetylgalactosamine (GalNAc) Oligonucleotide Conjugates
Oligonucleotides are predominantly hydrophilic species and require help in permeating cell membranes. One strategy to improve cellular uptake of therapeutic oligonucleotides is to conjugate them with non-toxic, lipophilic molecules. Glen Research offers products for cholesteryl, a-tocopheryl and stearyl labelling of oligonucleotides and this strategy has proved to be useful for delivering therapeutic oligonucleotides to a broad distribution of targets.
A more directed approach to the delivery of therapeutic oligonucleotides specifically to the liver has been to target the asialoglycoprotein receptor (ASGPR) using a suitable glycoconjugate. Indeed, ASGPR is the ideal target for delivery of therapeutic oligonucleotides to the liver since it combines tissue specificity, high expression levels and rapid internalization and turnover. The use of oligonucleotide glycoconjugates has led to significant advances in therapeutic delivery as evidenced by the work of Alnylam Pharmaceuticals which has developed multivalent N-acetylgalactosamine (GalNAc) conjugated siRNAs that bind at nanomolar levels to ASGPR.1 A similar strategy has been applied at Ionis Pharmaceuticals directed at the development of antisense oligonucleotide therapeutics.2
The GalNAc ligand originally used by Alnylam is shown in Figure 1. This so-called triantennary ligand would seem to lend itself to formation by post synthesis conjugation to the 3' terminus but a complex trivalent GalNAc support would also be perfectly applicable, if challenging to produce. However, an alternative approach3 using a monovalent GalNAc support with two additions of a monovalent GalNAc phosphoramidite was also described and yielded a structure shown in Figure 1. This (1+1+1) trivalent GalNAc structure led to GalNAc modified siRNA oligos with potency equal to the equivalent siRNA with the triantennary GalNAc ligand both in vitro and in vivo.
Figure 1: Structures of 3'-GalNac Modified Oligonucleotides |
|
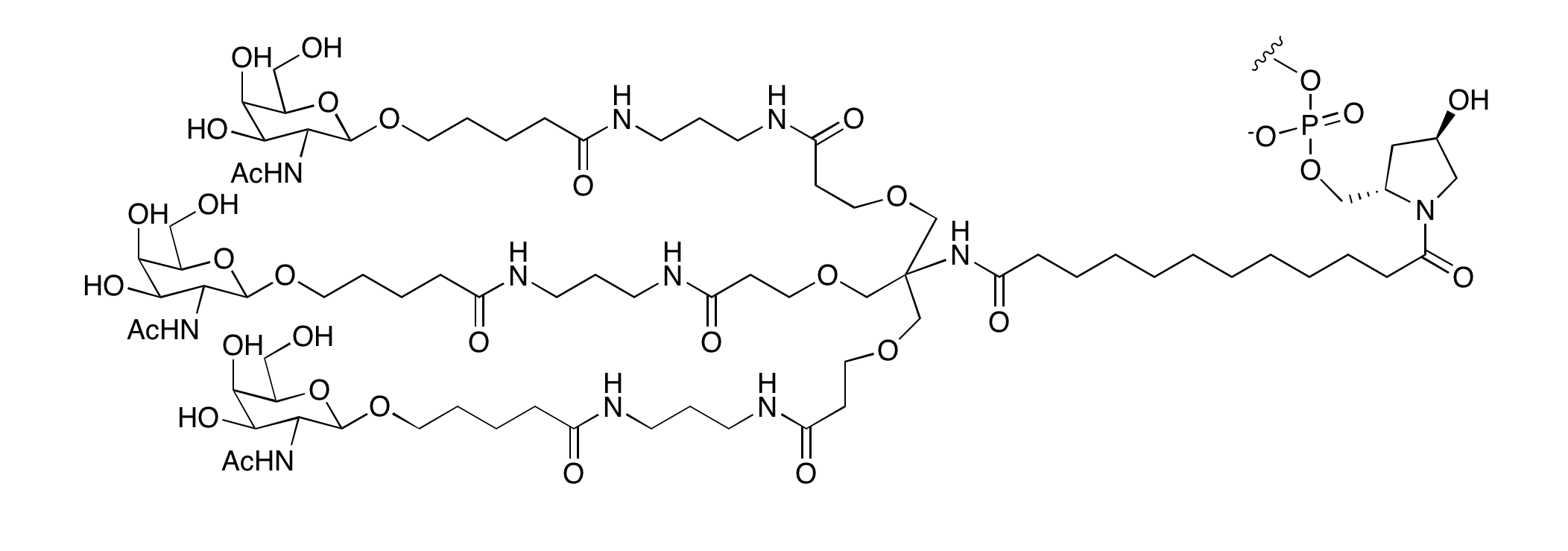 |
|
3'-triantennary GalNAc |
|
 |
|
3'-(1+1+1) trivalent GalNAc |
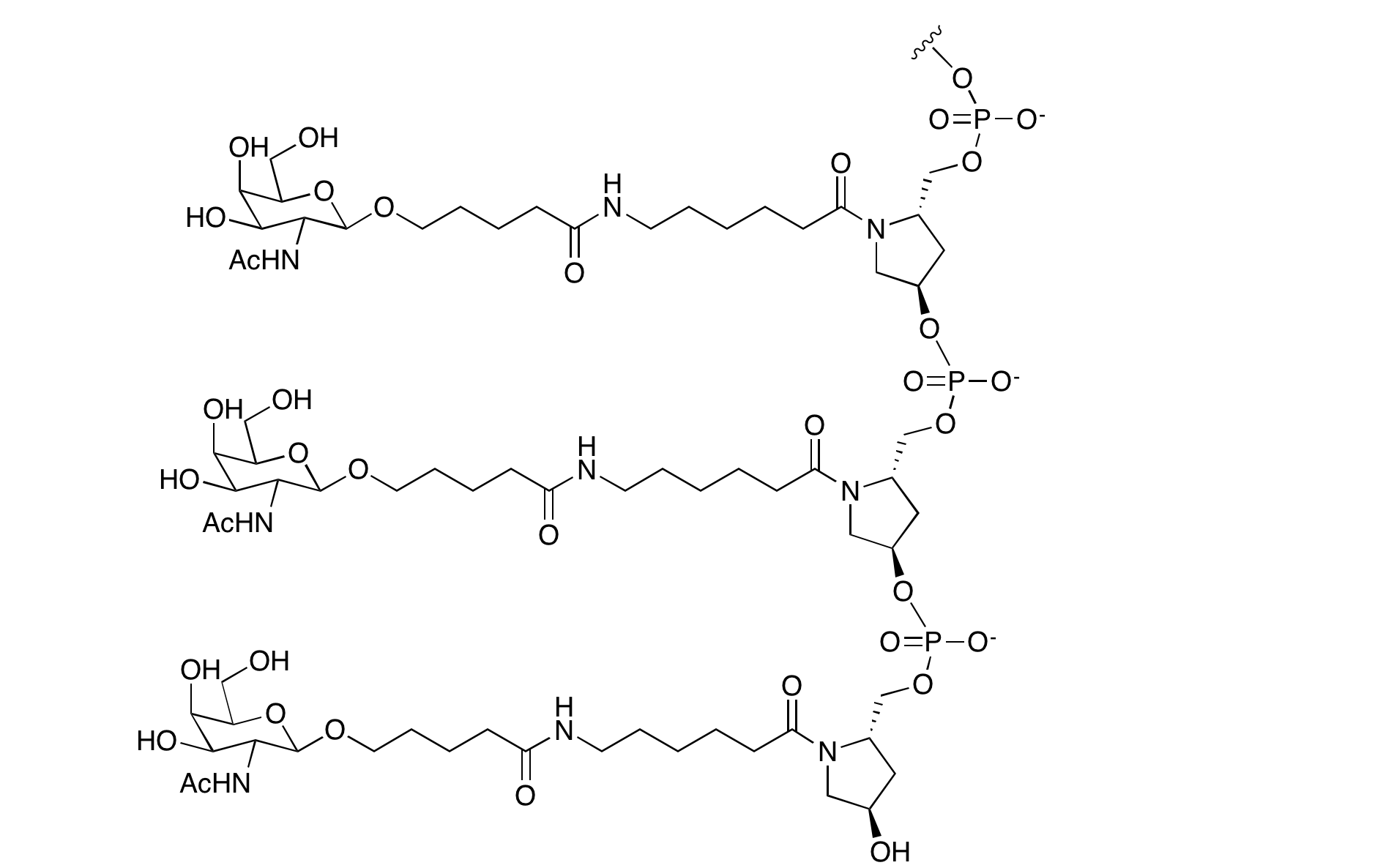
Researchers at Ionis have developed antisense oligonucleotides containing the GalNAc cluster. In their case, they were able to show2 that moving the triantennary GalNAc ligand to the 5' terminus led to improved potency in vitro and in vivo. As may be expected, such a large complex ligand lends itself to solution phase chemistry to produce GalNAc modified antisense oligos. However, a solid phase synthetic approach was also described, and compared to the solution phase approach.4 The structure of the 5'-GalNAc triantennary ligand is shown in Figure 2.
Figure 2: Structure of 5'-GalNac Modified Oligonucleotides |
 |
5'-triantennary GalNAc |
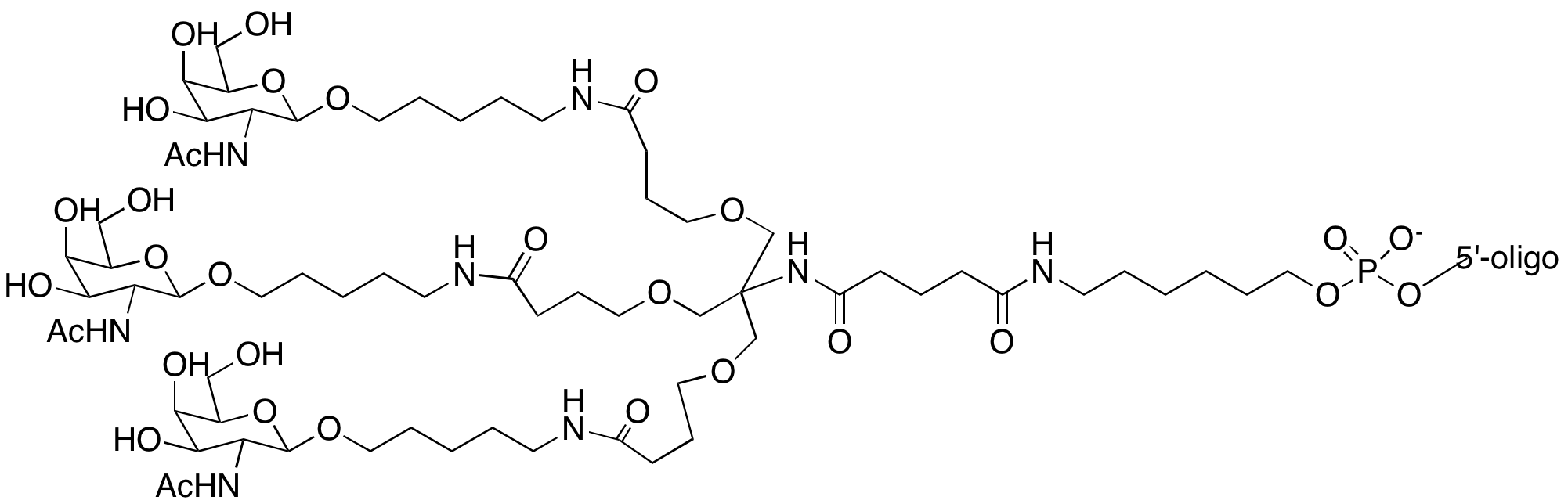
A further report on antisense oligonucleotides demonstrated5 the effectiveness of modifying at the 5' terminus using monovalent GalNAc ligands. Up to five GalNAc monomers3 were added in a serial manner (Figure 3) and it was shown that activity of the antisense oligonucleotides improved as the number of GalNAc units increased. The authors also showed that phosphodiester linkages between the GalNAc units were preferable to phosphorothioate linkages in their testing.5
Figure 3: Structure of Simple Monomer and GalNac Modified Oligonucleotides |
|
 |
 |
3' or 5'-(1+1+1) trivalent C3 Linker GalNAc |
5'-Simple GalNAc Phosphoramidite |
Glen Research is delighted to introduce a GalNAc modification strategy using a monomeric GalNAc support and the equivalent GalNAc phosphoramidite, as shown in Figure 4.
Figure 4: Structures of GalNac C3 Linker Phosphoramidite and CPG
 |
|
5'-C3 Linker GalNAc Phosphoramidite |
|
 |
|
C3 Linker GalNAc CPG |
|
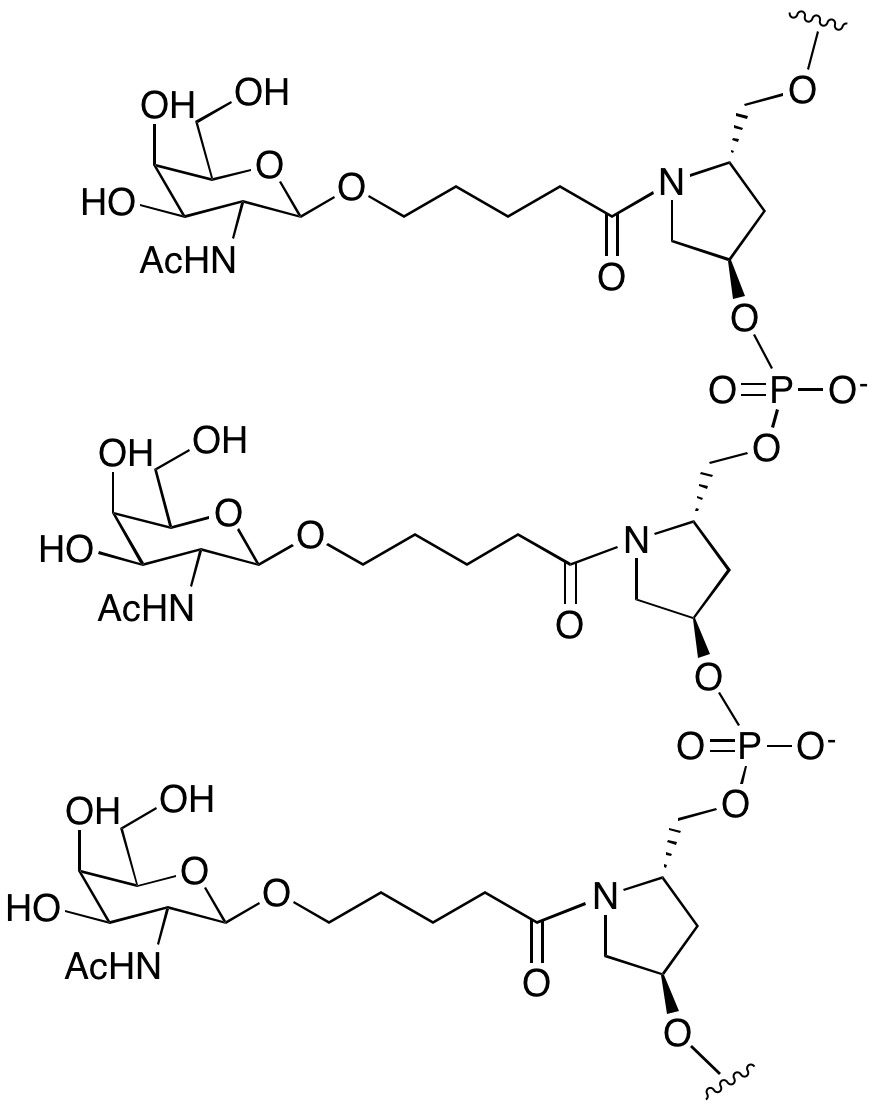
Figure 5: Structure of GalNac C3 Linker Modified Oligonucleotide |
 |
3' or 5'-(1+1+1) trivalent C3 Linker GalNAc |
The innovative linker in these products is a specialty of AM Chemicals LLC. At the core of the linker is a piperidine ring to which a 1,3-diol structure is attached. As we have noted before, 1,3-diol structures do not suffer the same level of elimination as 1,2-diol structures following synthesis and deprotection. Incorporating the piperidine ring in the structure also ensures that a chiral center is not formed when attaching a trityl group and a phosphoramidite to the two hydroxyl groups. In acyclic structures, the chiral center formed leads to diastereomers once an oligonucleotide is attached, which can lead to two product peaks of varying ratio in RP HPLC analysis. The piperidine structure removes this potential confusion of product peaks.
For this line of products, a trimethoxytrityl (TMT) group has been chosen instead of the more typical 4,4'-dimethoxytrityl (DMT) protecting group. In this structure, a DMT group is released more slowly than is typically seen on a 5'-hydroxyl of a nucleoside. The use of the more labile TMT groups ensures a rate of release equivalent to the regular DMT release. For those wishing to analyze the release of the TMT cation spectrophotometrically, the extinction coefficient at 482nm is 78,300 L/mol.cm.
Our experimental work has shown that these products are fully compatible with regular oligonucleotide synthesis and deprotection. A coupling time of 12 minutes is recommended for the phosphoramidite, while the support does not require any changes of cycle.
Oligonucleotides containing the GalNAc group can be deprotected using standard procedures during which the acetyl protecting groups on the GalNAc group are removed. The following conditions have been tested and shown to be acceptable:
Reagent |
Conditions |
|
Ammonium hydroxide |
65°C/2h |
|
55°C/17h* |
|
|
RT/2h |
|
|
* This is not optimal since low, but probably acceptable, levels of degradation were observed. |
|
|
Ammonium hydroxide/40% Methylamine(AMA) |
65°C/10 min |
|
Potassium Carbonate in Methanol |
RT/17h |
The chromatogram in Figure 6 demonstrates the high purity of 5'-GalNAc C3-T6 synthesized using a 12 minute coupling time. For the ESI spectrum (Figure 6, Inset), the target mass for the GalNAc C3-T6 is 2372.80 Da (Obs: 2372.7). Note: the higher molecular weight species with a mass of 2538.2 Da is the HFIPA adduct.
As shown in Figure 7, we have demonstrated that 5'-GalNAc C3 phosphoramidite can be used to prepare oligonucleotides with three consecutive GalNAc additions at the 5' terminus.
Glen Research is delighted to be able to offer these GalNAc C3 products under an agreement with AM Chemicals LLC. We thank Andrei Guzaev for helpful discussions while preparing this article to introduce these products for sale.
References
1. J.K. Nair, et al., J Am Chem Soc, 2014, 136, 16958-61.
2. T.P. Prakash, et al., Bioorganic & Medicinal Chemistry Letters, 2015, 25, 4127-4130.
3. K.G. Rajeev, et al., Chembiochem, 2015, 16, 903-8.
4. I. Cedillo, et al., Molecules, 2017, 22.
5. T. Yamamoto, M. Sawamura, F. Wada, M. Harada-Shiba, and S. Obika, Bioorganic & Medicinal Chemistry, 2016, 24, 26-32.
Figure 6: RP HPLC of 5'-GalNac C3 Linker Oligo |
Figure 7: RP HPLC of a 5'-(1+1+1) trivalent GalNac C3 Modified Oligonucleotide |
 |
 |
Product Information
5'-GalNAc C3 Phosphoramidite (10-1974)
- Glen Report 29.11: CDPI3 MGB™-Oligonucleotide Conjugates and Their Applications
- Glen Report 29.12: 5'-CDPI3 MGB™ Phosphoramidite and 3'-CDPI3 MGB™ CPG
- Glen Report 29.13: Studying DNA Repair Pathways Using Site-Specifically Modified Oligonucleotides
- Glen Report 29.14: N-Acetylgalactosamine (GalNAc) Oligonucleotide Conjugates
- Glen Report 29.15: Sequence Modification Using Glen Research's 5-Modified dU Family

