Glen Report 29.12: 5'-CDPI3 MGB™ Phosphoramidite and 3'-CDPI3 MGB™ CPG
As described in the preceding article by Eugene Lukhtanov of ELITechGroup Molecular Diagnostics, the tripeptide of dihydropyrroloindole-carboxylate (CDPI3) (Figure 1, Front Page) is a crescent-shaped molecule which binds isohelically within the B-form DNA minor groove. The reversible binding is mediated via hydrophobic and van der Waals interactions between the minor groove binder (MGB) and the floor of the groove. CDPI3 occupies a region of duplex DNA approximately 5 bases long.
DNA probes with conjugated MGB groups form extremely stable duplexes with single-stranded DNA targets, allowing shorter probes to be used for hybridization based assays. In comparison with unmodified DNA, MGB probes have higher melting temperature (Tm) and increased specificity, especially when a mismatch is in the MGB region of the duplex. MGB probes, therefore, can be significantly shorter than traditional probes, providing better sequence discrimination and flexibility to accommodate more targets.
The simplest approach to MGB probe design is to use an MGB support, add a quencher molecule as the first addition and complete the synthesis with a 5'-fluorophore. Alternatively, a fluorophore support could be used with the 5' terminus containing a quencher molecule followed by a final MGB addition at the 5' terminus.
Use of CDPI3 MGB
The iodine oxidation step during DNA synthesis cycles has the potential to damage minor bases and modifiers. So it was no surprise when it was found that the indole residues of CDPI3 MGB CPG are susceptible to iodination when standard 0.02 M Iodine oxidizer is used during synthesis. (This is only observed in the CDPI3 MGB CPG which lacks the ethoxycarbonyl protecting groups on the nitrogens of the indole rings of the 5'-CDPI3 MGB phosphoramidite.)
Figure 5: Chromatograms of 5’-T8-CDPI3 MGB-3' |
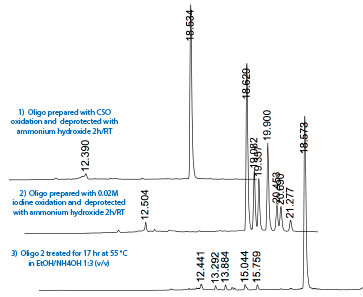 |
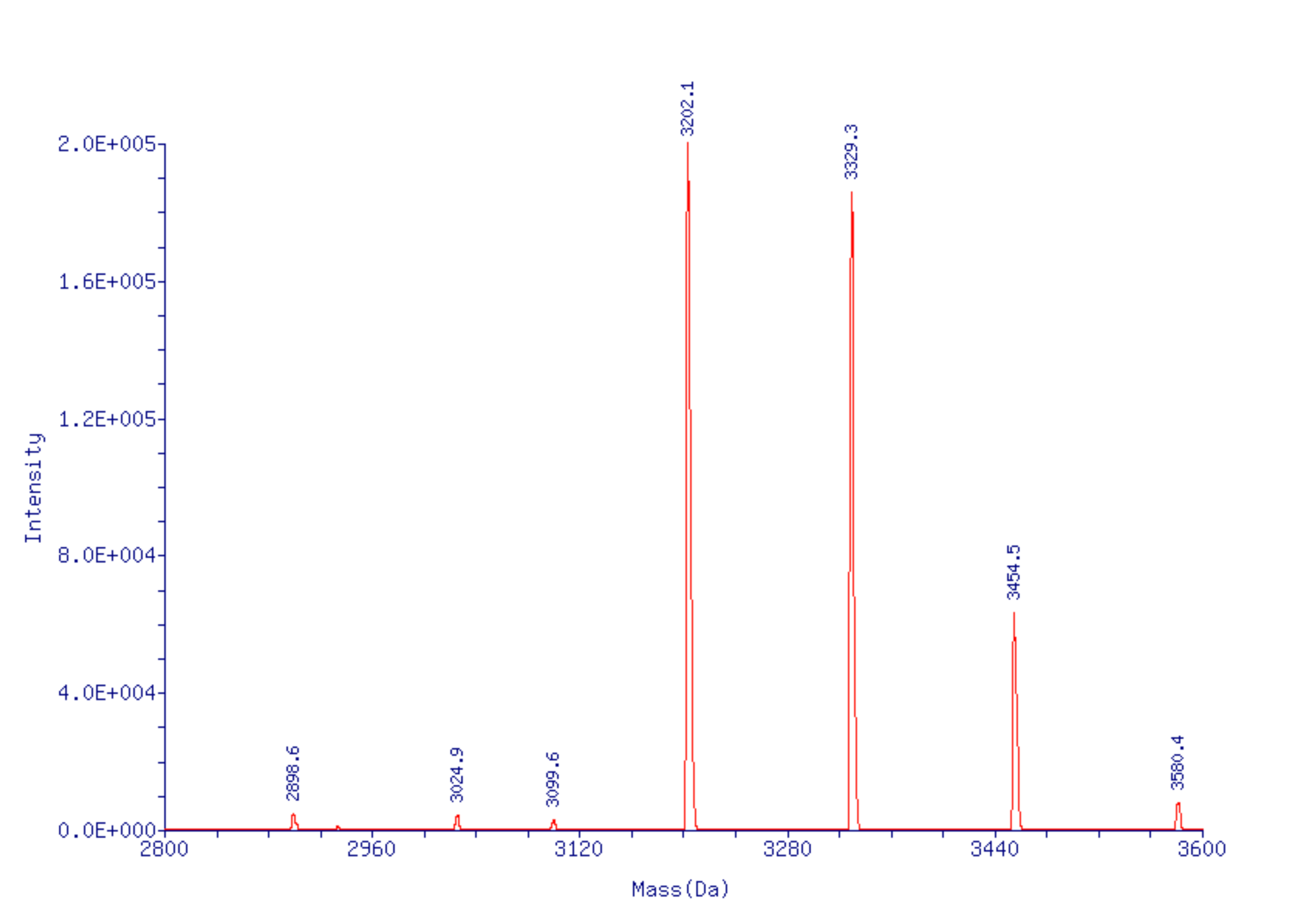
Chromatogram 1 shows the oligo prepared using CSO oxidation. Chromatogram 2 shows the result of iodine oxidation with the various permutations of 0, 1, 2 or 3 iodines coupled to the indoles of the CDPI3 MGB - as determined by ESI MS (Figure 6) - most likely at the 3 position of the indoles as described by Boger and Sakya J. Org Chem. 1992, 57, 1277-1284. Chromatogram 3 shows the oligo of Chromatogram 2 after deprotection with ethanolic ammonium hydroxide to reverse the iodination reactions. |
Figure 5 on Page 4 shows chromatograms 1) and 2) of the sequence 5'-T8-CDPI3 MGB-3' deprotected in 30% ammonium hydroxide for 2 hours at room temperature. The first oligo was synthesized using non-iodine oxidation with 0.5 M CSO and a 3 minute oxidation time while the second used 0.02 M iodine oxidizer. However, as shown in the third chromatogram, the iodination is reversible when the oligo is deprotected for 17 hr at 55 °C in EtOH/Nh4OH 1:3 (v/v).
Figure 6: ESI MS of Oligo 2, 5’-T8-CDPI3 MGB-3' |
 |
To determine how CDPI3 MGB fared with a more "real-world" oligo, 19mer oligonucleotides of the sequence,
5'-GCC TAA CTT CTG GAG ATG T- 3'
were synthesized with either a 3' or 5' CDPI3 MGB. The CDPI3 MGB phosphoramidite was found to be hydrophobic enough that it required 10% THF in ACN to go completely into solution at a 0.1 M concentration and required a 3 minute coupling time.
CDPI3 MGB Phosphoramidite
Diluent: 10% THF in ACN
Coupling time: 3 minutes
Oxidation: 0.02 M Iodine in THF/pyridine/water
Deprotection: EtOH/Nh4OH 1:3 (v/v) 17 hr at 55 °C
Purification: GlenPak™™ purified
Shown in Figure 7 is the chromatogram for the 5'-CDPI3 MGB probe after deprotection in EtOH/Nh4OH 1:3 (v/v) 17 hr at 55 °C and GlenPak™ purification.
With the CDPI3 MGB CPG, the optimum results are obtained if UltraMild monomers and Cap A are used during synthesis along with 0.5 M CSO oxidizer. However, the use of standard monomers with iodine oxidation followed by deprotection with EtOH/Nh4OH 1:3 (v/v) for 17 hr at 55 °C will give acceptable results.
CDPI3 MGB CPG
Coupling time: regular with UltraMil™™d monomers and Cap A used during synthesis
Oxidation: 0.5 M CSO in ACN (3 minute oxidation time)
Deprotection: 30% ammonium hydroxide 2 hr at room temperature
Purification: GlenPak™ purified
Figure 7: Chromatograms of 5’-CDPI3 MGB and 3’-CDPI3 MGB Probes |
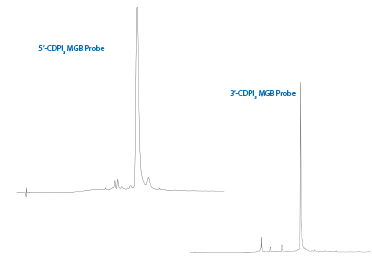 |
Also shown in Figure 7 is the chromatogram of 5'-GCC TAA CTT CTG GAG ATG T-CDPI3 MGB-3' after deprotection in 30% Nh4OH for 2 hours at room temperature and GlenPak™ purification. Given the hydrophobic nature of CDPI3 MGB, HPLC purification is preferred as the short failures containing the CDPI3 MGB are not efficiently removed by GlenPak™ purification.
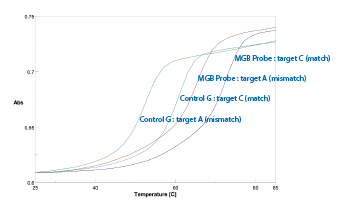
This oligo was used in a melting study comparing the Tm of the CDPI3 MGB-labeled probe against that of a control probe lacking the CDPI3 MGB. The probes were annealed to both matched (G-C) and mismatched (G-A) targets. As seen in the plot in Figure 8, a single incorporation of the CDPI3 MGB gave rise to an almost 12 °C increase in the melting temperature of the matched CDPI3 MGB probe compared to the unlabeled control. In addition, the selectivity - that is the ∆Tm (match - mismatch) - remained practically unchanged between the unlabeled control (8.1 °C) and the CDPI3 MGB-labeled oligo (7.0 °C) so the target specificity remains high despite the large increase in duplex stability.
Figure 8: melting study using the 3'-CDPI3 MGB-labeled probe |
 |
|
|
Tm (°C) |
|
MGB Probe : target C (match) |
72.0 |
|
Control G : target C (match) |
60.1 |
|
MGB Probe : target A (mismatch) |
65.0 |
|
Control G : target A (mismatch) |
52.0 |
|
|
|
|
ΔTm(MGB Probe - control): |
+11.9 °C |
|
|
|
|
ΔTm (match - mismatch) |
|
|
MGB Probe: |
-7.0 °C |
|
Control G: |
-8.1 °C |
We are pleased to add the 3'-CDPI3 MGB CPG and the 5'-CDPI3 MGB phosphoramidite to our repertoire of tools for increasing duplex stability, for use in PCR, DNA arrays, and for antisense applications. The structures are shown in Figure 9.
Starting on Page 13 of this newsletter, we review the sequence modifiers that might be useful in the design of MGB probes containing a fluorophore, quencher, etc., attached to the 5 position of dU.
Product Information
5'-CDPI3 MGB™ Phosphoramidite (10-5924)
- Glen Report 29.11: CDPI3 MGB™-Oligonucleotide Conjugates and Their Applications
- Glen Report 29.12: 5'-CDPI3 MGB™ Phosphoramidite and 3'-CDPI3 MGB™ CPG
- Glen Report 29.13: Studying DNA Repair Pathways Using Site-Specifically Modified Oligonucleotides
- Glen Report 29.14: N-Acetylgalactosamine (GalNAc) Oligonucleotide Conjugates
- Glen Report 29.15: Sequence Modification Using Glen Research's 5-Modified dU Family