Glen Report 26.23: New Product - 5'-Carboxy-Modifier C5
Conjugation reactions in organic chemistry are generally fairly straightforward with a nucleophile reacting with an electrophile to form the conjugate. In the field of oligonucleotide synthesis, it has proven to be expedient to include the nucleophile in the oligonucleotide for post synthesis conjugation with a suitable electrophile. There is a catalog of nucleophilic modifiers available to fit virtually any circumstances.
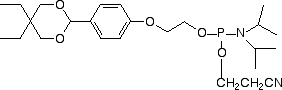
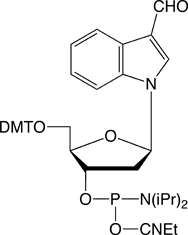
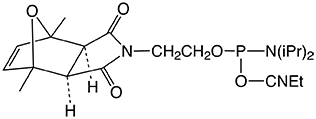
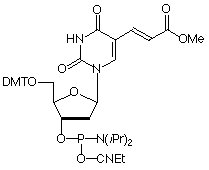

| Nucleophile | Target Electrophile |
|---|---|
| Amine | Activated Carboxylate |
| Thiol | Maleimide |
| Aminooxy | Aldehyde |
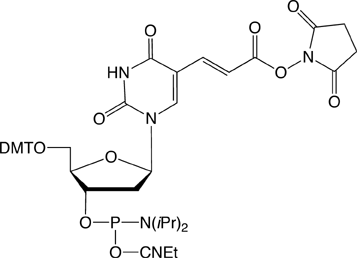
However, the electrophilic modifiers are a little more complicated. Although oligonucleotides are fairly easily modified with electrophilic aldehyde groups, which can readily be conjugated with hydrazide, hydrazine and aminooxy groups, our maleimide modifier requires some delicate chemistry to generate a maleimide-modified oligo in preparation for reaction with a thiol. And, in this article, we will focus on carboxy modification of oligonucleotides as we introduce a new 5'-Carboxy-Modifier.

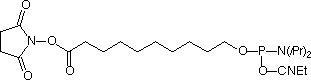
The simplest approach to carboxy modification of oligos is to include the carboxylate NHS ester in the phosphoramidite to form the protected and activated carboxylate in situ. Both of our NHS ester carboxy-modifiers (5'-Carboxy-Modifier C10 and NHS-Carboxy-dT) use this approach. While the oligo is still fully protected and attached to the support, it can be reacted with amines on the synthesis column. The reaction is fast and specific while any precious excess amine can be recovered. A downside to the approach of simple amine conjugation on column is that the reacted amino species must be able to survive the conditions of cleavage and deprotection.
If the amine chosen for conjugation is not stable to the conditions of cleavage and deprotection, the oligonucleotide has first to be cleaved and deprotected with sodium hydroxide to generate the carboxylate sodium salt. Deprotection with sodium hydroxide is a standard technique which is not very popular since the solution can not be simply evaporated like ammonium hydroxide or AMA. If ammonium hydroxide or AMA were to be used instead, the carboxylate NHS ester would be substantially converted to unreactive amide. Our Carboxy-dT also falls into this category in that the methyl ester protecting the carboxylate has to be hydrolyzed with sodium hydroxide.
An alternative approach to 5'-carboxy-modification was described1 a few years ago in which the carboxylate is protected with a 2-chlorotrityl group. This protecting group is simply removed using the standard deblock cycle to generate a free carboxyl group on an otherwise fully protected oligonucleotide. Using a standard peptide coupling reaction, the carboxyl group can be reacted with amines or amino acids to form conjugates. The procedure, shown in Figure 2, is also fully compatible with the formation of oligonucleotide peptide conjugates or libraries.

Alternatively if a 5' free carboxylate is desired, the oligonucleotide can be cleaved and deprotected trityl-on or trityl-off using ammonium hydroxide or AMA with no amide formation, as shown in Figure 3.
We have found that the optimal coupling time for this product is 3 minutes and the chlorotrityl group can be removed using the standard deblock procedure on the synthesizer. It should be noted that the chlorotrityl group is a different color from the regular DMT group so trityl monitors may not register the release properly.
| NHS Ester Protected | 2-Chlorotrityl Protected |
|---|---|
| Preactivated for On Column conjugation with simple amines | Requires trityl group to be removed and further activation |
| On Column conjugate must be stable to deprotection conditions | On Column conjugate must be stable to deprotection conditions |
| Isolation of free carboxylate requires deprotection with sodium hydroxide | Isolation of free carboxylate can be done using ammonium hydroxide, AMA or sodium hydroxide |
| No need to remove 2-chlorotrityl group prior to deprotection | |
| Useful for conjugation with amino acids and small peptides for library formation arboxy-Modifer C5 to extend our range of electrophilic modifiers. |
It should also be noted that the 2-chlorotrityl group is removed during oligo deprotection, as shown in Figure 3, and is incompatible with RP purification techniques.
We are happy to introduce 5’-Carboxy- Modifer C5 to extend our range of electrophilic modifiers.
Procedure for On-Column Conjugation
- Synthesize the 5'-carboxylate-modified oligo trityl-off and retain on the support.
- Pre-activate the carboxylic acid by treating the support-bound oligo with HATU (100 equivalents) and HOBT (100 equivalents) in dry DMF (100μL).
- Warm the reaction to 35°C and shake support for 35 minutes.
- After activation of the acid is complete, add triethylamine (100 equivalents) and the amine (100 equivalents).
- Warm the conjugation mixture to 35°C and shake the support for 1 hour.
- The unbound amine can easily be removed from the solid support by washing successively with DMF (2 x 100μL), ethanol (2 x 200μL), and distilled water (2x 200μL).
- The conjugate can then be deprotected and removed from the solid support using ammonium hydroxide or AMA using conditions appropriate for deprotection of the nucleobases.
- The conjugate is now ready for purification
Reference
- A.V. Kachalova, et al., Helv Chim Acta, 2002, 85, 2409-2416.
Product Information
- Glen Report 26.21: Reversible M6A RNA Modification
- Glen Report 26.22: New Product - N6-Me-A -Reversible M6A RNA Modification
- Glen Report 26.23: New Product - 5'-Carboxy-Modifier C5
- Glen Report 26.24: DNA-Mediated Charge Transport: A Novel Property of DNA with Diverse Applications
- Glen Report 26.25: New Product - Methylene Blue NHS Ester
- Glen Report 26.26: Technical Brief - Which 3'-Amino-Modifier?
- Glen Report 26.27: Technical Brief – Use of Click Chemistry for Possible Crosslinking, Click Synthesis of a Potentially Useful azido support and New Click Product - Cyanine 7 Azide
- Glen Report 26.28: New Product - Disulfo-Cyanine 7 Azide

