Glen Report 26.22: New Product - N6-Me-A -Reversible M6A RNA Modification
Since 2009, Glen Research has been active in providing monomers that may be implicated in the mechanisms of DNA methylation and demethylation. We were intrigued to see that a similar scenario may be playing out in the field of reversible RNA methylation and we were delighted when Chuan He and his colleague Qing Dai offered to review the field with specific emphasis on their own work at the University of Chicago.
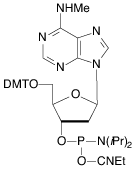
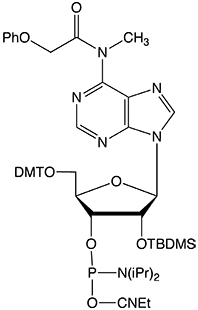
As they note in the accompanying article, it has been shown that the unprotected secondary amine of our original N6-Me-dA monomer (1) could support branching during oligonucleotide synthesis. In an article in The Glen Report 23.1 in 2011, we showed that although branching was minimal using tetrazole as activator, it became very significant (~15%) using DCI as activator. Consequently, we now offer the acetyl protected version of N6-Me-dA (2), which eliminates this branching reaction.
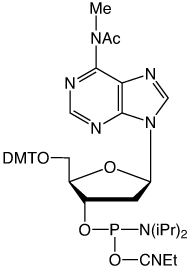
It was our decision to offer the N6-Me-A RNA monomer with a phenoxyacetyl protecting group to minimize potential branching. We have shown the phenoxyacetyl protected N6-Me-A (3) to be completely compatible with all popular RNA synthesis and deprotection methods, from UltraMild to the most popular procedure using AMA for deprotection.
Product Information
N6-Methyl-A-CE Phosphoramidite (10-3005)
- Glen Report 26.21: Reversible M6A RNA Modification
- Glen Report 26.22: New Product - N6-Me-A -Reversible M6A RNA Modification
- Glen Report 26.23: New Product - 5'-Carboxy-Modifier C5
- Glen Report 26.24: DNA-Mediated Charge Transport: A Novel Property of DNA with Diverse Applications
- Glen Report 26.25: New Product - Methylene Blue NHS Ester
- Glen Report 26.26: Technical Brief - Which 3'-Amino-Modifier?
- Glen Report 26.27: Technical Brief – Use of Click Chemistry for Possible Crosslinking, Click Synthesis of a Potentially Useful azido support and New Click Product - Cyanine 7 Azide
- Glen Report 26.28: New Product - Disulfo-Cyanine 7 Azide

