Glen Report 11.26: More Novel Monomers
As always, several new phosphoramidites and supports have made a break from our labs. Some never become products but other more enterprising candidates make it into The Glen Report. Here are several new products that we hope will brighten your research prospects.
EDTA-C2-dT
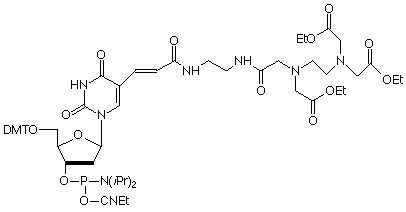
About time too! The Dervan group at Caltech described the cleavage of DNA with oligos containing EDTA-Fe at the C-5 position of Thymidine over 10 years ago.1 Their phosphoramidite contained the triethyl ester of EDTA and our version is shown, (1) in Figure 1. Coupling of EDTA-dT is normal but cleavage and deprotection should be carried out with sodium hydroxide in aqueous methanol (0.4M NaOH in methanol/water 4:1) overnight at room temperature.
The cleavage reaction is only initiated once Fe(II) and dithiothreitol are added and so is readily controlled. The Dervan group has described sequence-specific cleavage1 of single-stranded DNA by a probe containing EDTA, as well as cleavage2 of double-stranded DNA following triplex formation by the EDTA-oligo. A later publication described3 the application of the latter cleavage reaction to investigate the tertiary structure of RNA.
Product Information
EDTA-C2-dT-CE Phosphoramidite (10-1059)
7-Deaza-2'-deoxyXanthosine
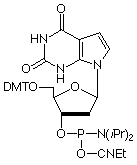
As a purine analogue of Thymidine, 7-deaza-2'-deoxyXanthosine (7-deaza-dX) promises to have interesting effects on DNA structure. Previously, 7-deaza-dX has been shown4 to form stable 7-deaza-X:A-T triplets, allowing triplex formation to occur in the anti-parallel motif. 7-Deaza-dX has also been investigated5 for its ability to form a non-standard base pair with a 2,4-diaminopyrimidine nucleoside analogue. In both cases, 7-deaza-dX was added to the sequence using H-phosphonate chemistry. We offer the phosphoramidite, as shown, (2) in Figure 1, which we have successfully incorporated into oligonucleotides. In a manner analogous to 7-deaza-dG, 7-deaza-dX requires the use of (10-camphorsulfonyl)oxaziridine (CSO)6 in place of the regular iodine oxidation if more than two insertions are to be made into an oligonucleotide.
Product Information
7-deaza-dX-CE Phosphoramidite has been discontinued
2'-Deoxypseudouridine
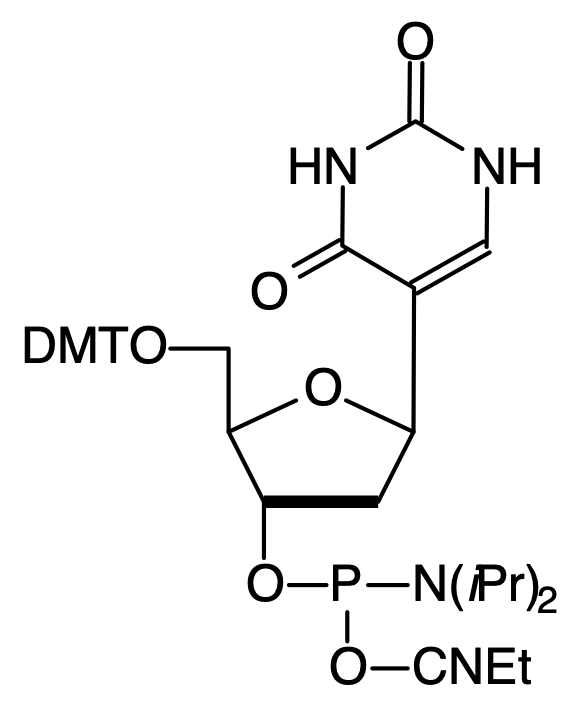
Another nucleoside analogue with the potential for affecting DNA structure is the C-nucleoside 2'-deoxypseudo-uridine. Again this analogue has been investigated7 for its behavior in triple helix formation and it has been shown to form stable triplexes with the 2'-deoxypseudouridine in the second strand (C:pseudoU-A triplets). Triplex formation was not observed in the same system when U was substituted for pseudoU. The phosphoramidite (3), Figure 1, is incorporated into oligonucleotides with no changes from standard procedures.
Product Information
2'-deoxypseudoU-CE Phosphoramidite (10-1055)
2'-OMe-5-Bromo-U
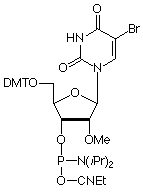
5-Bromo-2'-deoxyUridine (Br-dU) is the most actively used halogenated pyrimidine analogue in our catalog. Its uses range from crystallographic studies due to the heavy atom to cross-linking because of its photolability. We are happy to extend this action to the 2'-OMe-RNA series by the addition of 2'-OMe-5-bromo-U phosphoramidite (4) in Figure 1, to our product range.
Product Information
2'-OMe-5-Br-U-CE Phosphoramidite (10-3190)
Fluorescein Supports
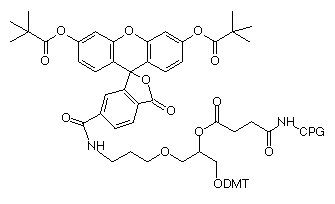

Our existing product, 3'-fluorescein CPG, has proved to be very useful for insertion of a fluorescein group at the 3'-terminus of an oligonucleotide. Indeed the use of 3'-fluorescein CPG is probably the simplest way to prepare a fluorescein-labelled oligonucleotide. However, a significant drawback has been the difficulty in producing a really pure oligonucleotide from this support. Several mechanisms, and probably the structure itself, lead to several fluorescein-containing species which in turn lead to multiple peaks on RP HPLC. It is difficult to explain to a customer that several isomers exist in this oligo and all these peaks are "the product". But when researchers moved on to labelling at the 3' terminus with fluorescein and the 5' terminus with a second dye, purification became truly messy.
We now offer two fluorescein supports to help clarify the situation. Both are derivatives of 6-carboxyfluorescin (6-FAM) and are single isomers. Both use an amide linkage which is stable during cleavage and deprotection and does not allow isomer formation. 3'-(6-FAM) CPG, (5) in Figure 1, effectively blocks the 3'-terminus from polymerase extension as well as exonuclease digestion, while fluorescein-dT CPG, (6) in Figure 1, allows both of these enzymatic activities to proceed. We believe these two supports are the perfect complements for our existing 5'-fluorescein (6-FAM) phosphoramidite for 5'-terminal labelling and fluorescein-dT for internal labelling.
Product Information
3'-(6-FAM) CPG (20-2961)
3'-Fluorescein-dT CPG (20-2056)
References
G.B. Dreyer and P.B. Dervan, Proc Natl Acad Sci U S A, 1985, 82, 968-72.
H.E. Moser and P.B. Dervan, Science, 1987, 238, 645-50.
H. Han and P.B. Dervan, Proc Natl Acad Sci U S A, 1994, 91, 4955-9.
J.F. Milligan, S.H. Krawczyk, S. Wadwani, and M.D. Matteucci, Nucleic Acids Res., 1993, 21, 327-333.
M.J. Lutz, H.A. Held, M. Hottiger, U. Hubscher, and S.A. Benner, Nucleic Acids Res, 1996, 24, 1308-13.
Glen Report, 1996, 9, 8-9.
T.L. Trapane, M.S. Christopherson, C.D. Roby, P.O.P. Ts'O, and D. Wang, J. Am. Chem. Soc., 1994, 116, 8412-8413.
- Glen Report 11.21: Advances in RNA Synthesis and Structural Analysis
- Glen Report 11.22: TOM-Protecting-Group™ - A Major Improvement in RNA Synthesis
- Glen Report 11.23: New Columns for Expedite and LV Applications
- Glen Report 11.24: Nucleotide Analogs for Interference Mapping of RNA
- Glen Report 11.25: Alternatives to Expedite Monomers
- Glen Report 11.26: More Novel Monomers

