Fluorescein is a bright green dye first synthesized by Adolf von Baeyer in 1871. Since then, it has been used in a wide range of applications including leveling equipment, optometry and water tracing. It was even used to turn the Chicago River green for St. Patrick’s Day celebrations for several years.
For our customers, fluorescein is more commonly known as the most popular fluorophore for oligonucleotide labeling as well as biomolecular labeling in general. The molecule has excitation and emission wavelengths of 495 and 521 nm, respectively, and is conveniently excited by the 488 nm spectral line of an argon-ion laser. With a very high quantum yield, fluorescein is one of the brighter small molecule dyes available. In the context of oligonucleotide synthesis, fluorescein has the added advantage of being very stable to the basic environments that are necessary during oligonucleotide deprotection, which is not always the case for fluorophores. Deprotection of such oligonucleotides is covered in more depth in a separate article in this issue.1
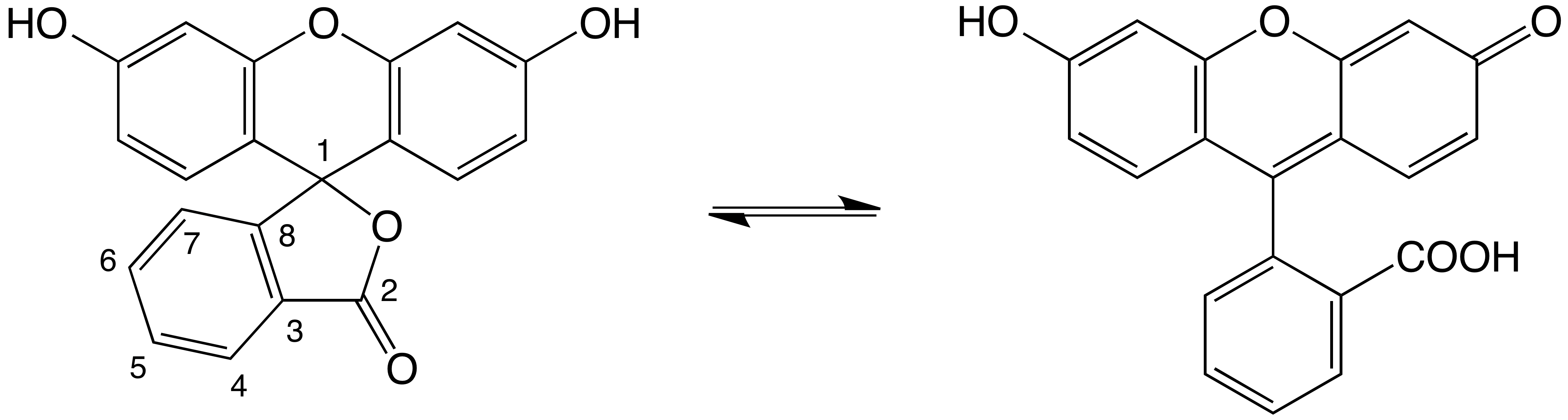
Glen Research carries many versions of fluorescein, currently five phosphoramidites, five supports, one NHS ester and one azide. A couple of these are based on 5-fluorescein isothiocyanate (5-FITC) while the rest are derived from 6-carboxyfluorescein (6-FAM). The number denotes the attachment point of the linker based on the interesting numbering system of fluorescein (Figure 1). For those interested in how one decides which phosphoramidite or synthesis support to use, this topic was reviewed earlier.2
Figure 1. Fluorescein structure
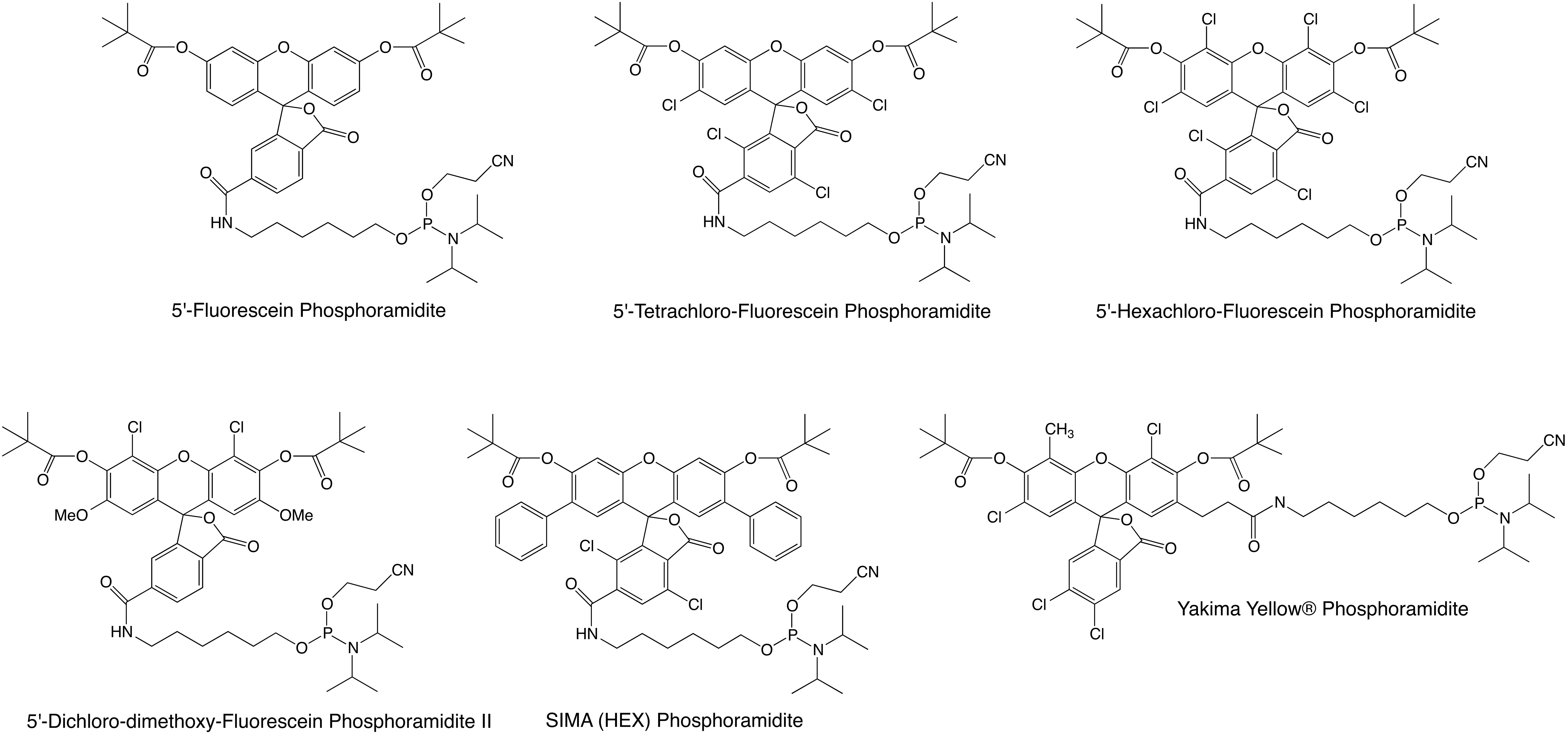
Glen Research also carries derivatives of fluorescein. These contain substitutions on the aromatic rings that red-shift the spectral properties of the fluorophore for researchers that require multiple wavelength/dye applications (Table 1). They include 6-carboxy versions of tetrachloro-fluorescein (TET), hexachloro-fluorescein (HEX), dichloro-dimethoxy-fluorescein (JOE) and dichloro-diphenyl-fluorescein (SIMA). It should be noted that SIMA is a much more deprotection stable substitute for HEX. We also have Yakima Yellow®, a non-symmetrical tetrachloro-bisalkylated-fluorescein. A comparison of their structures based on the 5’-phosphoramidites reinforces how similar they are (Figure 2). In case one was wondering, each of the fluoresceins and its derivatives are protected by 2 pivaloyl groups on the aromatic hydroxyls that render the molecule non-fluorescent. It is only when these groups are released during deprotection that the expected color and emission of the dyes are realized.
Table 1. Spectral properties of fluorescein and its derivatives
|
|
Absorbance Maximum (nm) |
Emission Maximum (nm) |
Color |
|
Fluorescein |
494 |
525 |
Green |
|
Tetrachloro-Fluorescein |
521 |
536 |
Orange |
|
Hexachloro-Fluorescein |
535 |
556 |
Pink |
|
Dichloro-dimethoxy-Fluorescein |
525 |
548 |
Orange/Pink |
|
SIMA (HEX) |
538 |
551 |
Pink |
|
Yakima Yellow® |
530 |
549 |
Yellow
|
Fluorescein and its related derivatives continue to be very popular fluorophore labels for oligonucleotides and represent a significant portion of our fluorophore portfolio. In future articles, we will review other major families of fluorophores that we also carry.
Figure 2. 5’-Fluorescein phosphoramidite and its derivatives
References
The Glen Report, 2022, 34.2, 1.
The Glen Report, 2016, 28.2, 5.
Product Information