In previous Glen Report articles, we have demonstrated that the iodine oxidation step during DNA synthesis cycles has the potential to damage some minor bases and modifiers. In this article, we have compiled some of the previous information to show that (1S)-(+)-(10-camphorsulfonyl)-oxaziridine (CSO) (1) is an ideal non-aqueous oxidizer for oligonucleotide synthesis.
 |
 |
| Figure 1: Structures of CSO and DBCO-dT | |
Iodine-based oxidizers have been the standard for DNA and RNA synthesis since the advent of automated synthesizers. They are fast and efficient oxidizers, typically requiring less than 30 seconds for complete oxidation of phosphite triesters to phosphate triesters. However, while iodine-based oxidizers work well for most applications, there are some circumstances where non-aqueous oxidizers may be advantageous, especially where the bases or linkages being produced are sensitive to the presence of water and/or iodine during synthesis.
Non-aqueous oxidizers, typically peroxides, including tert-butyl hydroperoxide, cumene hydroperoxide, hydrogen peroxide, and bis-trimethylsilyl peroxide, among others, have also been employed in DNA synthesis. These peroxides tend to be unstable, requiring that they be freshly formulated just prior to use, and so are difficult to use in routine automated synthesis, hence the need for a stable, effective non-aqueous oxidizer.
In 1996, we investigated the use of (1S)-(+)-(10-camphorsulfonyl)-oxaziridine (CSO) as a non-aqueous oxidizer for the synthesis of oligonucleotides containing methyl phosphonate linkages. It was already known that the use of low water oxidizers improved the synthesis of oligos containing methyl phosphonates. However, we were able to show that a 0.5M solution of CSO in acetonitrile (40-4632-xx) gave exellent results in the synthesis of chimeric oligonucleotides containing methyl phosphonate linkages.
Some bases that show instability to multiple successive synthesis cycles exhibit better stability with a low water oxidizer. At the same time as investigating methyl phosphonates, we also found that a 0.5M solution of CSO in acetonitrile worked well as an oxidizer for the synthesis of oligos containing multiple incorporations of 7-deaza-dG, compared with iodine oxidation which caused substantial degradation.
Recently a customer noted difficulty in preparing oligo-dI. Because of the known sensitivity of purine bases to iodine oxidation, we suggested using 0.5M CSO (with a 3 minute oxidation time) instead of the standard Iodine based oxidizer. Using CSO, the customer found that the synthesis was much improved and the oligo was successfully isolated.
Since Inosine is somewhat susceptible to damage by iodine during oxidation, we now recommend the use 0.5M CSO in anhydrous acetonitrile with a 3 minute oxidation time, If there are >6 incorporations of inosine within a sequence.1
In a recent article,2 we described the sensitivity of the copper-free click reagent DBCO to iodine oxidation. The occurrence of a side reaction came to our attention3 during the earlier investigation of a customer problem preparing a relatively long oligo (63-mer) containing DBCO-dT (2). The customer’s report seemed to indicate that the DBCO moiety was being cleaved during repetitive synthesis cycles. Conceptually, this was unexpected since amide linkages are resistant to hydrolysis, which implied that DBCO-dT is sensitive to one or more of the synthesis reagents and that the repeated exposure during the synthesis of long oligos led to cleavage of the DBCO.
To test this hypothesis, CPG from a simple 12-mer dT synthesis containing three additions of DBCO-dT was used. The RP HPLC of the test oligo synthesis is shown in Figure 2a. This oligo was then subjected to treatment with standard DNA synthesis oxidizer, 0.02 M Iodine, for 5 minutes at room temperature. This exposure is equivalent to roughly 20 synthesis cycles. As shown in Figure 2b, the resulting degradation was quite dramatic.
a. Test oligo after regular synthesis and deprotection |
b. Test oligo treated for a further 5 minutes with 0.02M iodine oxidizer |
|
|
|
Figure 2: RP HPLC of Test 12-mer containing three DBCO-dT additions |
|
As described above, with other analogues with sensitivity to iodine, we have achieved good results using CSO. So, when a 63-mer was re-synthesized using 0.5 M CSO in acetonitrile and a 3 minute oxidation time, the hoped-for improvement was very significant indeed.
Figure 3 shows the deconvolved electrospray MS data for the same sequence synthesized using standard 0.02 M Iodine versus 0.5 M CSO with the target mass being 20,511 Da. It is clear that the DBCO moiety is being cleaved when exposed to iodine-based oxidizers. What appears to have occurred during oxidation with iodine is the formation of an N-iodo amide, making the amide linkage unstable. During deprotection, the DBCO is eliminated, leaving a hexamido linker present. (The splitting of the -DBCO peaks is 14 Da, indicating the formation of both the amide and N-methylamide linkers which results from the oligo being deprotected in AMA). The lower molecular weight peaks associated with the CSO-oxidized oligo are deletion mutants (-1, -2 and -3 dTs), which suggests the oxidation time of 3 minutes should have been increased slightly for an oligo of this length.
63-mer prepared using standard 0.02M iodine oxidation6 |
63-mer prepared using oxidation with 0.05M CSO in acetonitrile6 |
 |
|
Figure 3: MS analysis of 63-mer containing three DBCO-dT additions |
|
As a result of these data, we now recommend that synthesis of oligos containing DBCO-dT be completed using 0.5 M CSO oxidizer. Acceptable results can be achieved with iodine oxidation if DBCO-dT is subjected to no more than 8-10 further cycles.
As with methyl phosphonates, the PACE modification is degraded by N-methylimidazole during capping and is susceptible to cleavage during aqueous oxidation using iodine. For this reason, we recommend using Unicap (40-4410-XX), a phosphoramidite-based capping reagent, and 0.5 M CSO (40-4632-XX), a non-aqueous oxidizer, for best results.4
Following coupling of the 2’-OMe PACE monomer, the recommended procedure is to cap using Unicap with a regular coupling time and then oxidize using 0.5 M CSO for 3 minutes.
As noted, the iodine oxidation step during DNA synthesis cycles has the potential to damage minor bases and modifiers. So it was no surprise when it was found that the indole residues of CDPI3MGB CPG (Figure 4 on Page 7) are susceptible to iodination when standard 0.02 M Iodine oxidizer is used during synthesis. (This is only observed in the CDPI3MGB CPG which lacks the ethoxycarbonyl protecting groups on the nitrogens of the indole rings of the 5’-CDPI3MGB phosphoramidite.)
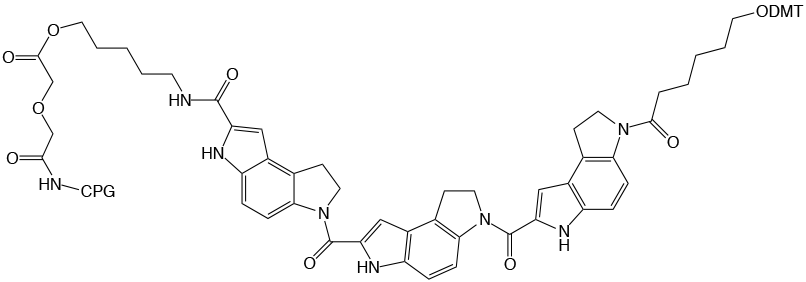 |
Figure 4: Structure of CDPI3 MGB™ CPG |
Figure 5, Page 7 shows chromatograms 1) and 2) of the sequence 5’-T8-CDPI3MGB-3’ deprotected in 30% ammonium hydroxide for 2 hours at room temperature. The first oligo was synthesized using non-iodine oxidation with 0.5 M CSO and a 3 minute oxidation time while the second used 0.02 M iodine oxidizer. Chromatogram 2) illustrates the multiplicity of iodination on the indole rings. However in this case, as shown in the third chromatogram, the iodination is mostly reversible when the oligo is deprotected for 17 hr at 55 °C in EtOH/NH4OH 1:3 (v/v).5
1) Oligo prepared with CSO oxidation and deprotected with ammonium hydroxide 2h/RT |
|
2) Oligo prepared with 0.02M iodine oxidation and deprotected with ammonium hydroxide 2h/RT |
|
3) Oligo 2 treated for 17 hr at 55 °C in EtOH/NH4OH 1:3 (v/v) |
|
Chromatogram 1 shows the oligo prepared using CSO oxidation. Chromatogram 2 shows the result of iodine oxidation with the various permutations of 0, 1, 2 or 3 iodines coupled to the indoles of the CDPI3MGB - as determined by ESI MS - most likely at the 3 position of the indoles as described by Boger and Sakya J. Org Chem. 1992, 57, 1277-1284. Chromatogram 3 shows the oligo of Chromatogram 2 after deprotection with ethanolic ammonium hydroxide to reverse the iodination reactions. |
|
Figure 5: Chromatograms of 5’-T8-CDPI3MGB-3’ |
|
In this review article, we have demonstrated several examples of situations where side reactions in minor bases and modifiers are essentially eliminated by the use of a non-aqueous and non-iodine containing oxidizer. While these side reactions are relatively minor in the case of simple oligonucleotides with a single addition of the minor base or modifier, multiple additions and/or multiple further cycles of oligonucleotide synthesis revealed extensive modification by iodine. Our advice is to consider the use of the non-aqueous oxidizer CSO when unusual and unexpected results manifest themselves in the synthesis of more complex or longer oligonucleotides.
1. The Glen Report, 2018, 30.1, 13.
2. The Glen Report, 2018, 30.1, 4.
3. The Glen Report, 2015, 27.1, 10.
4. The Glen Report, 2018, 30.1, 9.
5. The Glen Report, 2017, 29.1, 4.
0.10M CSO in Anhydrous Acetonitrile 40-4631